- Poly(azomethine) Based Micelles for Delivery of Caffeic Acid Phenethyl Ester
Nermin Saliha Topuzogullari, Fatma Sayan Poyraz, Murat Topuzogullari*, and Banu Mansuroglu†

Department of Molecular Biology and Genetics, Faculty of Arts and Sciences, Yildiz Technical University, 34220, Türkiye
*Department of Bioengineering, Faculty of Chemical and Metallurgical Engineering, Yildiz Technical University, 34220, Türkiye- Cafferic Acid Phenethyl Ester 전달을 위한 Poly(azomethine) 기반 마이셀 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
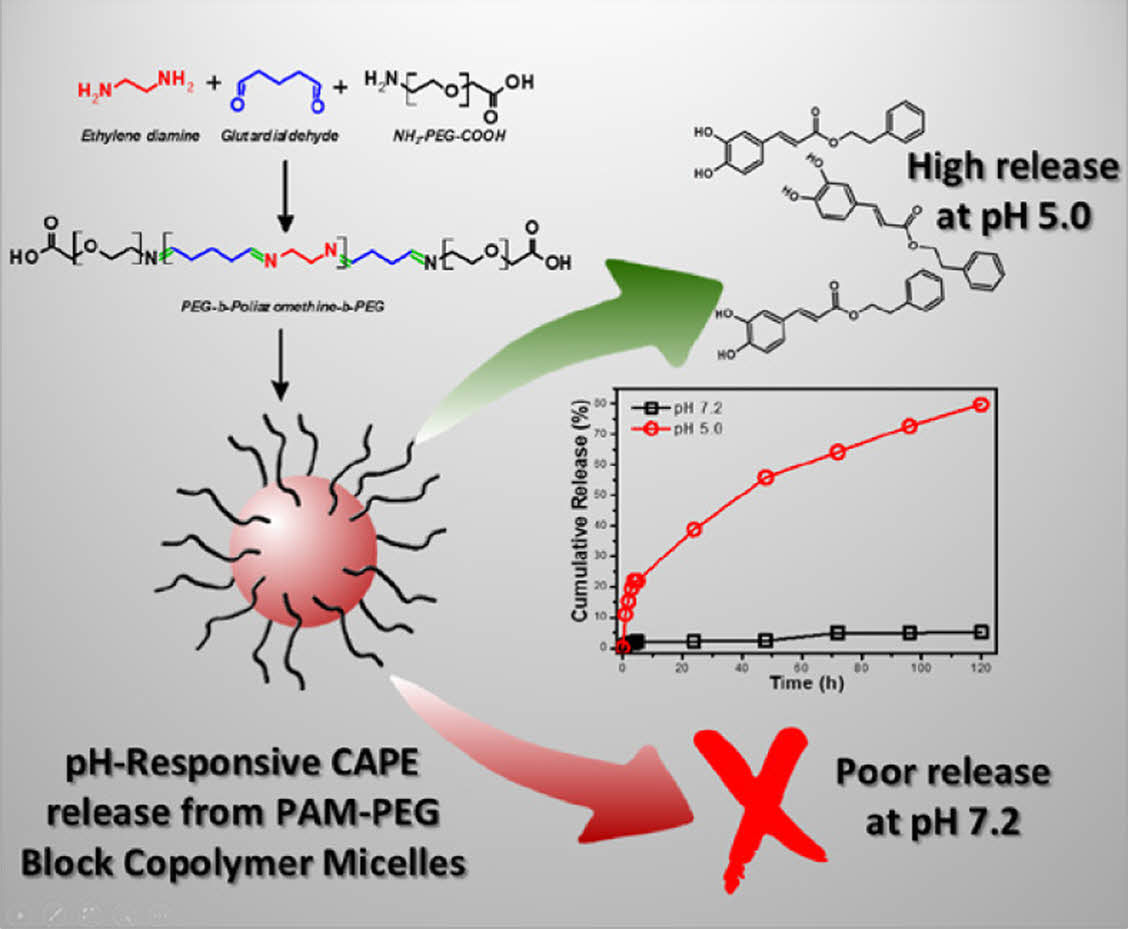
Poly(azomethine) based micelles have been developed for the delivery of caffeic acid phenethyl ester (CAPE). These micelles, synthesized from a polyazomethine-PEG block copolymer, demonstrate significant potential in drug release systems due to its pH-sensitive biodegradable nature. Characterization through SEM revealed spherical micelles with an average diameter of 135.5 nm. The micelles' drug release behavior was studied at physiological (pH 7.2) and lysosomal (pH 5.0) pH levels. The findings indicated pH sensitive release of CAPE from the micelle of which only 2.14% of CAPE was released at pH 7.2 while 38.8% of CAPE was released at pH 5.0 after 24 h. In vitro cell viability assays using human-derived fibroblast cells showed that CAPE-loaded micelles exhibited minimal toxicity after 48 hours compared to CAPE and free micelle. These results suggest the potential of polyazomethine-based micelles as efficient carriers for targeted drug delivery for therapeutic purposes.
This study introduces caffeic acid phenethyl ester (CAPE)-loaded polyazomethine-poly(ethylene glycol) (PEG) micelles, synthesized and characterized for targeted drug delivery. These micelles demonstrate pH-responsive controlled release of CAPE in which 2.14% of the encapsulated CAPE was released at pH 7.2 after 24 hours, while the cumulative CAPE release reached to 38.8% after 24 hours at pH 5.0. The innovative use of polyazomethine-PEG copolymers shows promising biocompatibility and efficacy to be used in drug delivery applications.
Keywords: drug delivery, micelle, pH-sensitive, polyazomethine, polyimine.
We would like to acknowledge the financial support provided to Nermin Saliha Topuzogullari through the 100/2000 YÖK Ph.D. Scholarship Program from Council of Higher Education Republic of Türkiye (YÖK).
The authors declare that there is no conflict of interest.
Biodegradable polymers offer a significant advantage as biomaterials because they decompose and are eliminated from the body after fulfilling their purpose, making their temporary use in medical applications. These polymers exhibit a broad spectrum of applications, notably serving as nanocarriers, surgical sutures, and implants. This diverse family of polymers can be both naturally derived and synthetically produced.1 Biodegradable polymers such as polylactides, polycaprolactones, polyurethanes, polyphosphazenes, polyanhydrides, polyacetals, polyamides, poly(ortho esters), polyphosphoesters, and polycarbonates are utilized in various fields including drug delivery, vaccine applications, tissue engineering, and prosthetics.2 Among these polymers, polyazomethines (PAMs) are less commonly known and used.
PAMs contain the –CH=N– functional group in the main chain, which is sensitive to pH values.3,4 Schiff bases are stable in neutral and alkaline environments, but they hydrolyze in low pH values by breaking the double bond, resulting in their decomposition into the amine and carbonyl compounds that form them. It can be considered that such an imine structure completely dissociates in an aqueous solution at a pH value below 6.5. This reversibility allows PAMs to be used as pH-sensitive biomaterials in biological systems.3 The sensitivity of PAM polymers to acidic pH enables controlled drug release in specific organs (stomach, intestinal system, or vagina) or intracellular organelles (endosomes or lysosomes).5
PAMs are generally produced by the condensation reaction of dialdehydes and diamines. Monomers containing both aldehyde and amine groups can also be used. Various studies have been conducted on PAMs in different fields. Saçak and colleagues reported that the polymer poly(3-[[4-(dimethylamino) benzylidene]amino]phenol) (P(3-DBAP)), a Schiff base polymer containing a phenol group, showed thermal stability up to 1200 ℃ and exhibited antibacterial and antifungal activity in both monomer and polymer forms.4 In another study, a Schiff base-based pH-sensitive polyimine imidazole-4,5-imine (PSI) polymer was synthesized, resulting in a cationic polymer.6 By forming a complex with siRNA, the pH sensitivity of the polymer allowed for the release of siRNA from endosomes to the cytoplasm. A cross-linked polymer was obtained through the reaction of a polyamine molecule with a fructose-derived dialdehyde.7 In a separate study, porous nanoparticles were produced through the Schiff base reaction of a phenolic dialdehyde with a polyamine molecule.8 Ma and colleagues synthesized a biocompatible and pH-sensitive copolymer by linking the poly(2-ethylacryloyloxyethyl phosphorylcholine) block and PLA block with a benzoyl imine linkage, demonstrating its use as a paclitaxel carrier.9 The rapid cleavage of the benzoyl imine linkage at acidic pH values resulted in the disintegration of the micellar structure, thereby accelerating the release of paclitaxel.
One of the most significant disadvantages of PAMs is their low solubility. However, the addition of a hydrophilic block such as poly(ethylene glycol) (PEG) to PAM intended for use as a biomaterial increases its aqueous presence and adds biocompatibility. The amphiphilic structure formed by adding a PEG block to the PAM structure can assemble in an aqueous solution to form micellar particles with a hydrophilic shell and a hydrophobic core.10,11 Polymeric micelles increase the aqueous availability of drugs, can be produced at the nanoscale, and can protect the cargo molecule from the immune system.12 These features of polymeric micelles allow for prolonged circulation in the bloodstream, passive targeting through the enhanced permeability and retention (EPR) effect, and reduced adverse side effects of drug molecules.13 While the PAM block in the polymer structure remains stable at neutral or basic pH values, it will dissociate into its monomers under acidic conditions, releasing the drug. At the cellular level, the acidity of endosomes (pH ~5-6) and their fusion with lysosomes (pH ~4-5) present an important pH gradient for effective intracellular drug delivery from such system.5
CAPE is a naturally occurring bioactive compound derived from honeybees, having diverse biological and pharmacological properties of antiviral, antioxidant, anti-inflammatory, antibacterial, and anticancer activities.14,15 Despite its therapeutic potential, CAPE's application is limited by its poor water solubility due to its hydrophobic nature, highlighting the need for an effective delivery system.
Despite the presence of many biodegradable drug carrier systems, there are no polyazomethine-PEG-based polymeric micelle carrier systems. Furthermore, the synthesis of this polymer under mild conditions is one of its most important advantages. In this study, the synthesis of PAM-PEG block copolymers using two fundamental molecules such as ethylenediamine and glutardialdehyde, the production of nanoscale drug delivery systems from these polymers, and the investigation of their biological activity were aimed. The model cargo molecule used was CAPE that needs to be delivered for an efficient therapeutic activity. The produced polymer and micellar structures were characterized, and their biological activities were investigated through in vitro tests.
Materials. Ethylene diamine and glutardialdehyde (25%) were purchased from Merck. Amine and carboxylic acid functionalized PEG (NH2-PEG-COOH) (Mw: 2 kDa) was obtained from Creative PEGworks. All solvents were used as received.
Human-derived fibroblast (HDF) cells used for the cytotoxicity assay were from the American Type Culture Collection (ATCC). Dulbecco’s modified eagle medium (DMEM), Fetal Bovine Serum (FBS), Antibiotics, L-glutamine and all the chemicals used in cell culture studies were purchased from Gibco. (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) was obtained from BioBasic.
Synthesis of PAM-b-PEG Copolymer. To synthesize the PAM-PEG block copolymer, 2×10-3 mole (134 µL) of ethylene diamine and 2×10-3 mole (800 µL) of glutardialdehyde were added into 4 mL of ethanol. Then, 2×10-5 mole (40 mg) of NH2-PEG-COOH was added into the solution. Following a 24-hour period of continuous stirring at ambient temperature, an excess of diethyl ether was introduced to the solution to induce polymer precipitation. The polymer obtained was dried under vacuum overnight.
Preparation of PAM-b-PEG Micelles. PAM-PEG copolymer micelles were prepared using the dialysis method.16
Briefly, 25 mg PAM-PEG copolymer was dissolved in 100 mL of DMF. This solution was then transferred into a dialysis membrane with a molecular weight cut-off of 14000 g/mol and dialyzed against 1200 mL of ultrapure water under magnetic stirring for 24 h. The dialysis water was refreshed every 6 h. The resultant clear solution was subsequently lyophilized and stored at ambient temperature.
CAPE loaded micelles were produced using the same method by dissolving the polymer and CAPE together in DMF using the mass ratio of mCAPE/mPAM-PEG:1/5.
Characterizations. FTIR spectra of the polymer and the micelles were acquired from Perkin Elmer Spectrum One FTIR (with ATR apparatus) spectrophotometer. Shimadzu UV-1700 UV-Vis spectrophotometer was used for absorbance measurements during the drug release study. NMR spectra were recorded by Bruker Ascend 500. Particle size, polydispersity index and zeta potential values were obtained from Malvern Zetasizer NanoZS dynamic (DLS) and electrophoretic (ELS) light scattering spectroscopy at 25 ℃. The micelles' morphological features were examined using a Thermo Scientific Quattro S scanning electron microscope with a scanning transmission electron microscopy (STEM) detector operating at 25–30 kV under high vacuum. For imaging, the samples were placed on a carbon film-coated copper grid and dried.
To assess the encapsulation efficiency and loading capacity of the micelles, the amount of non-encapsulated CAPE remaining in the solution outside the dialysis membrane was quantified. The concentration of free CAPE was measured using a calibration curve derived from the absorbance at 325 nm.
The encapsulation efficiency (EE) and drug loading (DL) of micelles were determined according to the Equations 1 and 2, in which mCAPE-EN and mCAPE-I are the encapsulated and initial amount of CAPE, respectively, while mT is the total amount of lyophilized CAPE-loaded micelle.
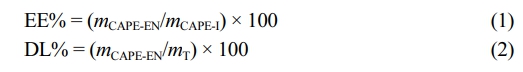
In Vitro Release Studies. In vitro release studies for the CAPE-loaded micelles were conducted at both pH 7.2 and pH 5.0. Specifically, 1.0 mg of the prepared micelles was dispersed in 1 mL of phosphate-buffered saline (PBS) at pH 7.2 and dialyzed against 5 mL of PBS at pH 7.2, under incubation at 37 ℃ with shaking at 100 rpm. At designated intervals, 1 mL of the dialysate was collected and replaced with an equal volume of fresh PBS. The collected samples were analyzed for CAPE release using a UV-Vis spectrophotometer at 325 nm.
The in vitro release study was also conducted at pH 5.0 using acetate buffer in place of PBS.
Biodegradation Studies. CAPE-loaded micelles were dispersed as 1 mg/mL in both PBS (pH 7.2) and acetate buffer (pH 5.0), respectively. The solutions were incubated at 37 ℃ with shaking at 100 rpm. At designated intervals, the samples were analyzed with DLS at 37 ℃ to determine derived count rates.
Cell Culture Conditions. Human-derived fibroblast (HDF) cells were cultured in accordance with ATCC guidelines. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 µg/mL streptomycin, 100 units/mL penicillin, and 0.2 mM L-glutamine. The cultures were incubated at 37 ℃ in a humidified atmosphere containing 5% CO2. The culture medium was replaced every two to three days.
Cell Viability Assay. MTT assay was used for determination of cell viability. HDF cells were seeded to 96 well plates as 1×104 cells/mL. Following the incubation, the medium was changed with fresh medium containing a series of dilutions (0-1 mg/mL) of CAPE, CAPE-Micelle (containing 0-1 mg/mL CAPE) and free Micelle. After 24 and 48 h of incubation, medium was removed from each well and MTT reagent was added and incubated at 37 ℃ for 3 h. Subsequently, the MTT solution was harvested, and the formazan crystals, produced by the activity of mitochondrial dehydrogenase enzymes, were dissolved in 0.1 mL of DMSO. Absorbance was then measured at 570 nm using a microplate reader. The cells, which received no treatment, served as the control. Metabolic activity values are presented as the mean ± SD of a minimum of three replicates. Cell viability (%) was determined using Equation 3, in which ODT and ODUT are the absorbance values of the treated and untreated (control) cells, respectively.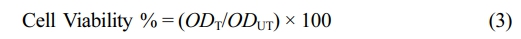
The study aims to present the potential of polyazomethines in drug release systems as biodegradable polymers. For this purpose, we synthesized a block copolymer of polyazomethine with PEG and prepared CAPE loaded micelles using this block copolymer. In the first step of the study, we synthesized the PAM-PEG block copolymer using condensation polymerization shown in Scheme 1, in which PEG quantity was adjusted to be at the molar ratio of nmonomer/nPEG = 100/1.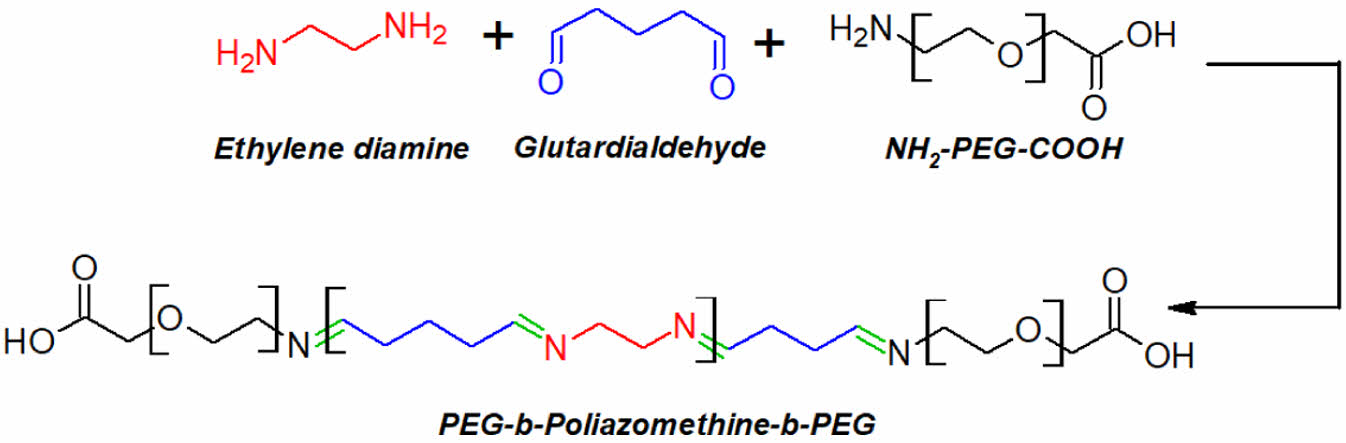
Scheme 1. The synthesis reaction of the PAM-PEG block copolymer.
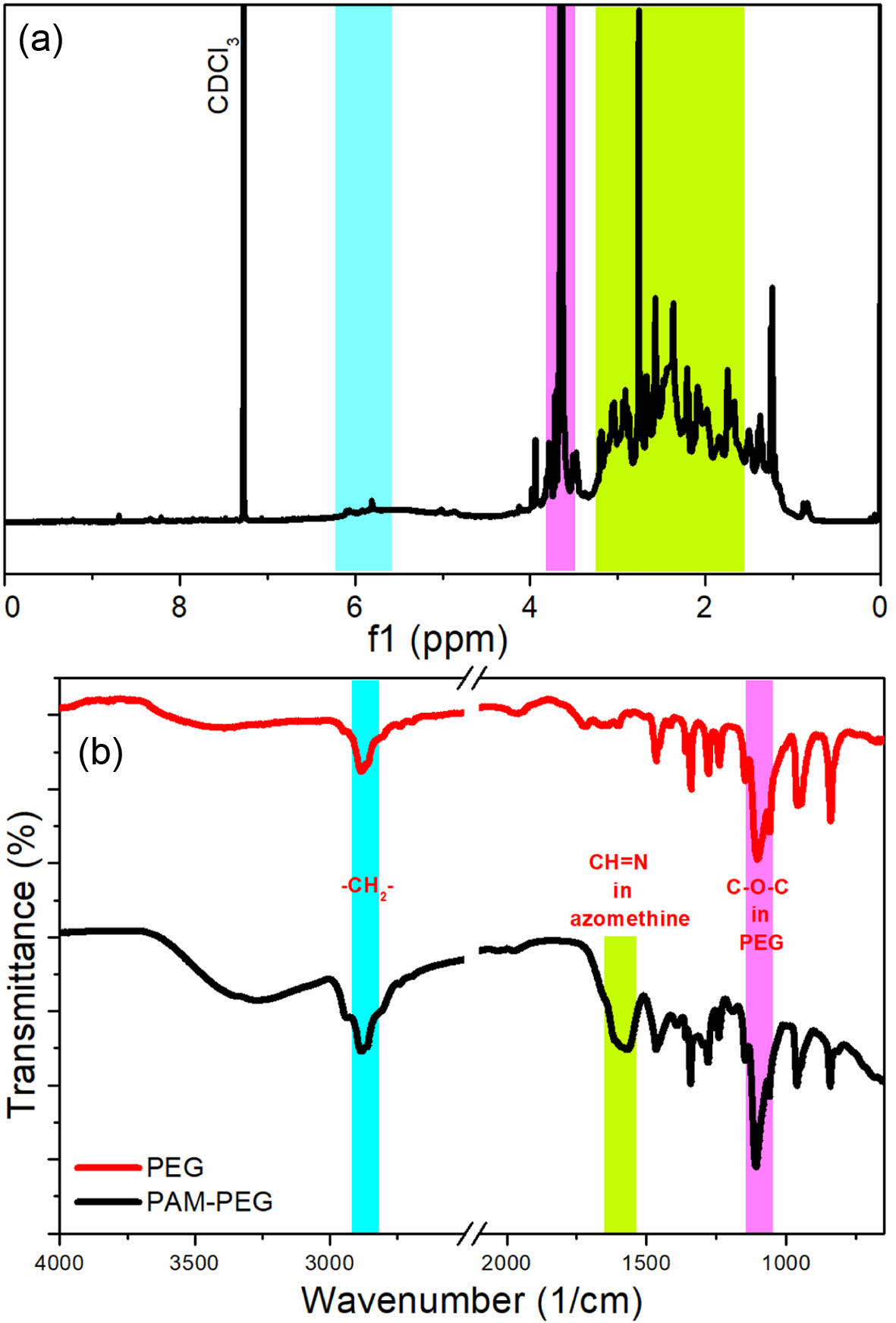
The produced copolymer was analyzed with NMR and FTIR spectroscopies and the acquired spectra are given in Figure 1. In 1H NMR spectrum of the copolymer, protons of PEG (-CH2CH2O-) are observed at 3.64 ppm. The peaks observed between 6.06 and 5.81 ppm may belong to the protons of imine (Schiff base) protons (-CH=N-). The protons of azomethine groups are observed mostly above 8 ppm especially for the aromatic polyazomethines of which imine groups are directly connected to the aromatic rings.17 In the produced copolymer, the azomethine groups are linked with aliphatic groups, which could be the reason that azomethine groups are observed at slightly lower values in 1H NMR spectrum. The peaks lower than 3.2 ppm corresponds to the protons of aliphatic carbons between imine groups.
The molecular weight of the copolymer was determined using NMR spectroscopy. The extremely low solubility of the copolymer in solvents commonly used in GPC, such as water, THF, and DMF, limited us to using NMR spectrum for this purpose. As a result, the Mn value of the PAM block obtained from NMR spectroscopy was calculated to be 1125 g/mol. Given that the Mn value of the PEG molecule used is known to be 2000 g/mol, the molecular weight of the PAM-PEG block copolymer was determined to be 3125 g/mol.
In FTIR spectra, the similar bands observed at 1110 and 2883 cm-1 in both spectra correspond to the C-O-C stretching of aliphatic ethers and the CH stretching of aliphatic CH2 groups, respectively, present in both PEG and the synthesized copolymer. The wide band between 1640 and 1520 cm-1 is intersection of two bands having minimum points at 1615 and 1568 cm-1. The band at 1615 cm-1 corresponds to the stretching of -N=CH- group, which is shown in several previous studies.18,19 The band at 1568 cm-1 also belongs to the -N=CH- group according to Kamaci et al.18
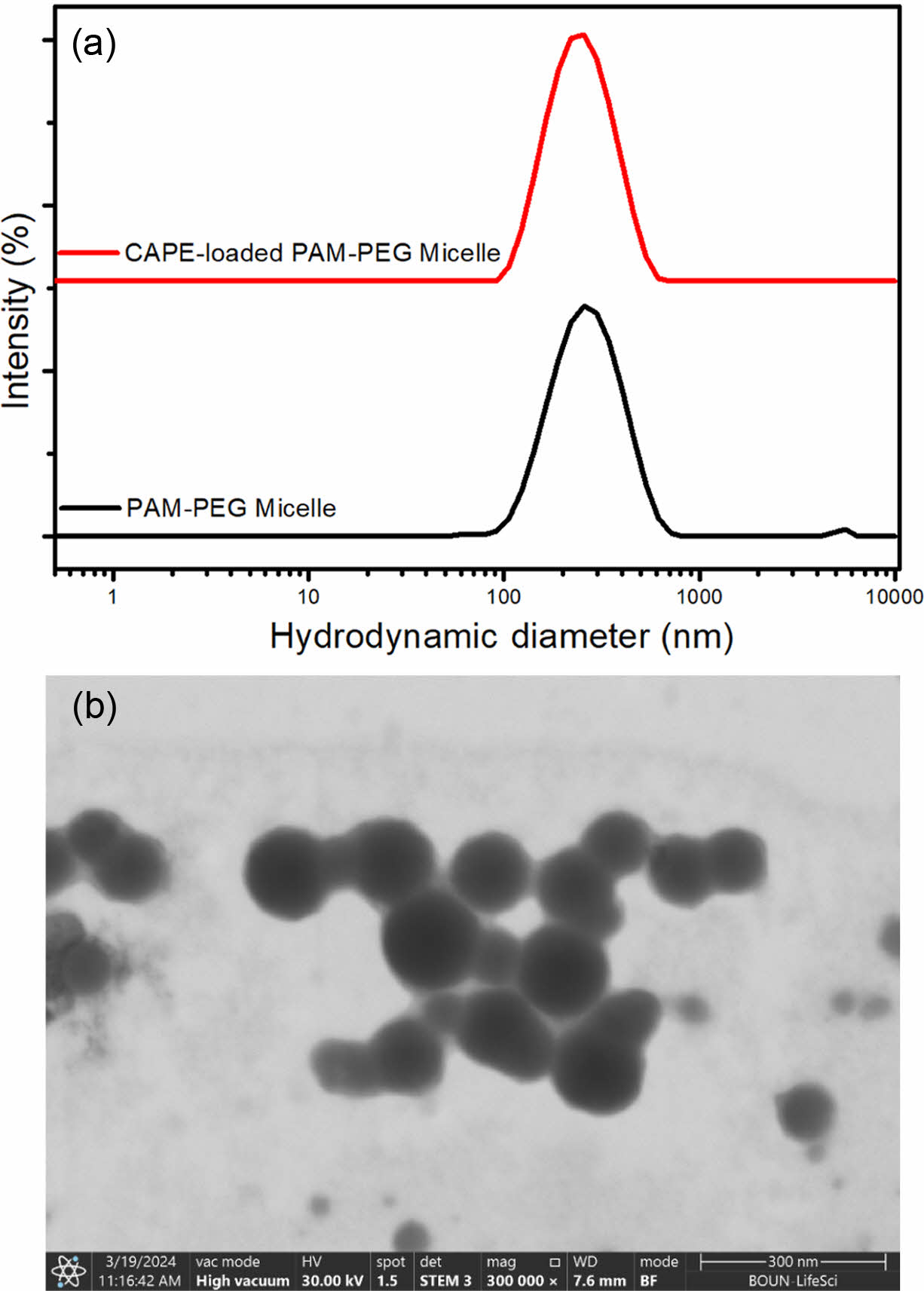
After the synthesis of the copolymer, it was used to prepare micelles to deliver CAPE. The empty and CAPE-loaded PAM-PEG copolymer micelles were prepared by dialysis technique. Figure 2 gives the size distributions of these micelles acquired from dynamic light scattering technique. The z-average hydrodynamic diameters of free and CAPE-loaded micelles were 259.6 and 233.8 nm, respectively, while the PDI values were lower than 0.25 for both micelles. The decrease in micelle size is likely due to the enhanced hydrophobic interactions between the PAM block and CAPE molecules, which result in a more compact micelle core. No specific chemical interactions were detected between CAPE and the polymer, as confirmed by FTIR analysis.
The zeta potential of the micelles were measured using the ELS. The results showed that the surface charge was slightly negative at pH 7.2, with zeta potential values of and -5.11 mV for free micelles and -7.49 mV for CAPE-loaded micelles. This negative surface charge is likely attributed to the chain-end COOH group of the PEG used in the synthesis of the copolymer. During the synthesis, the NH2 terminal group of PEG participates in the formation of the –CH=N– bond, leaving the COOH group unbound. Additionally, this COOH group is located on the side of the micelle exposed to the aqueous phase, leading to the observed negative charge.
The micelles were visualized with SEM to investigate their morphology. The micelles exhibited spherical shape and an average diameter of 135.5 nm, which is smaller than the diameter obtained from DLS. The size reduction observed in SEM analysis compared to the DLS results can be attributed to the collapse and dehydration of the core and shell of the micelle particles during the SEM sample preparation process.20
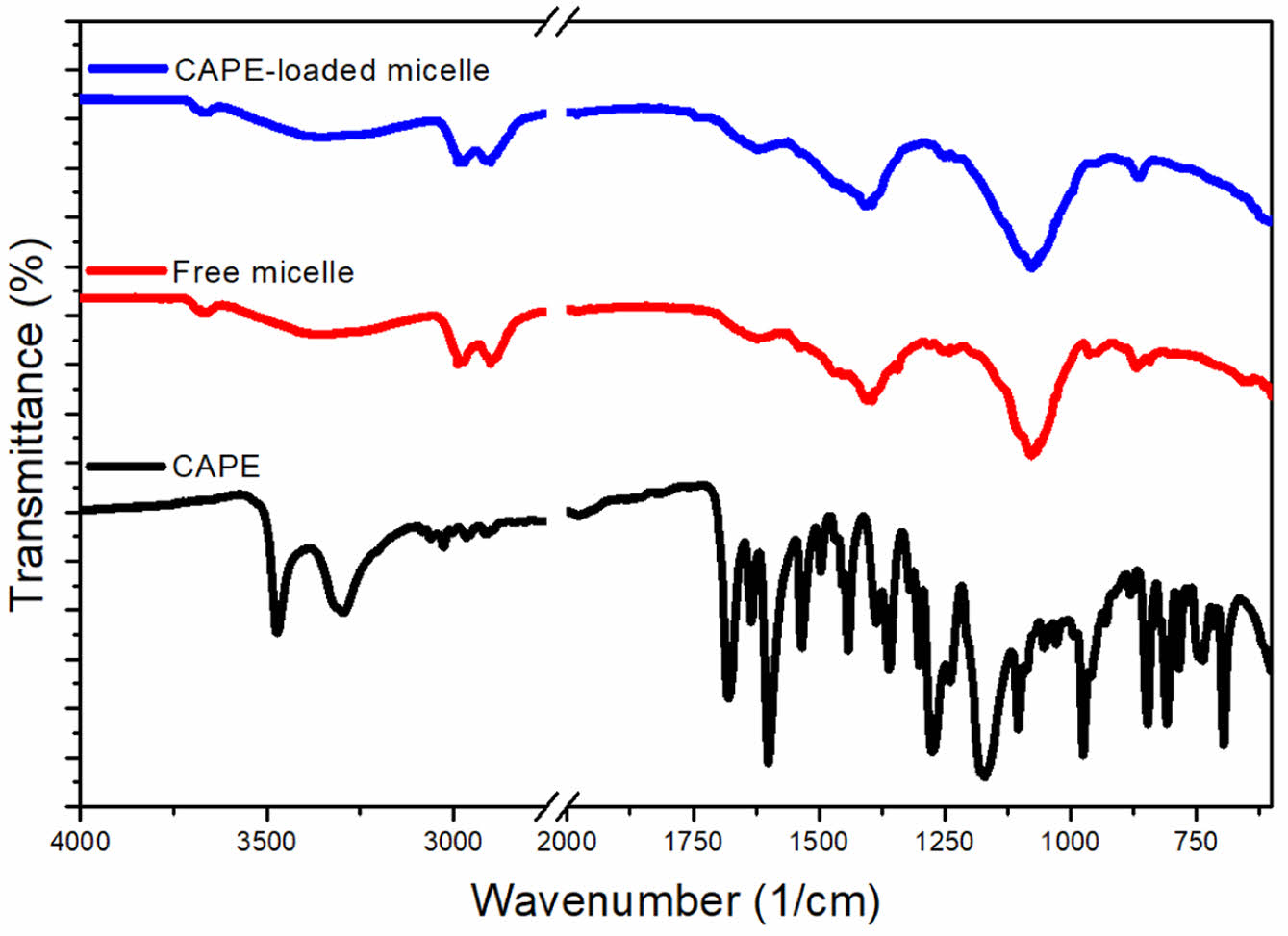
By using indirect method, the drug loading capacity of the micelle was determined. The drug loading of CAPE-loaded micelles was found to be as low as 3%, which is a known disadvantage of micelles.21 Subsequently, the micelles were characterized using FTIR spectroscopy to assess the adsorbed CAPE molecules. Figure 3 exhibits the FTIR spectra of free and CAPE-Loaded micelles. The CAPE molecule exhibits characteristic bands at 1679 and 1601 cm-1, corresponding to the C=O and C=C bonds, respectively, and a band at 1275 cm-1 corresponding to the C-O-C group in the ester bond.22 The FTIR spectra of both the free and CAPE-loaded micelles displayed similar features, with the CH=N group observed at 1650 cm-1 and ether (C-O-C) groups at 1077 cm-1. Additionally, bands at 2901 and 2987 cm-1 indicated aliphatic CH stretching of CH2 groups. In the spectrum of the CAPE-loaded micelles, the characteristic bands of CAPE were not observed, representing that the drug molecules were encapsulated within the core of the micelles rather than adsorbed on the surface. However, the fact that the peak of CAPE encapsulated within the micelle core is not observed, while the peak of the -CH=N- group located in the same micelle core is visible, is likely due to the very low drug loading ratio of 3% and the -CH=N- units being highly abundant as repeating structural units. Moreover, the spectra showed no evidence of chemical interaction between the drug and the polymer.
Following the characterization of the CAPE-loaded micelles, their release behavior was examined at pH 7.2 and 5.0. The neutral pH was selected to simulate physiological conditions, while the acidic pH was chosen to mimic lysosomal environments and cancerous tissues.23,24 The release quantity of the CAPE molecules was determined using the dialysis technique, and UV spectrometry was employed to detect the released CAPE.
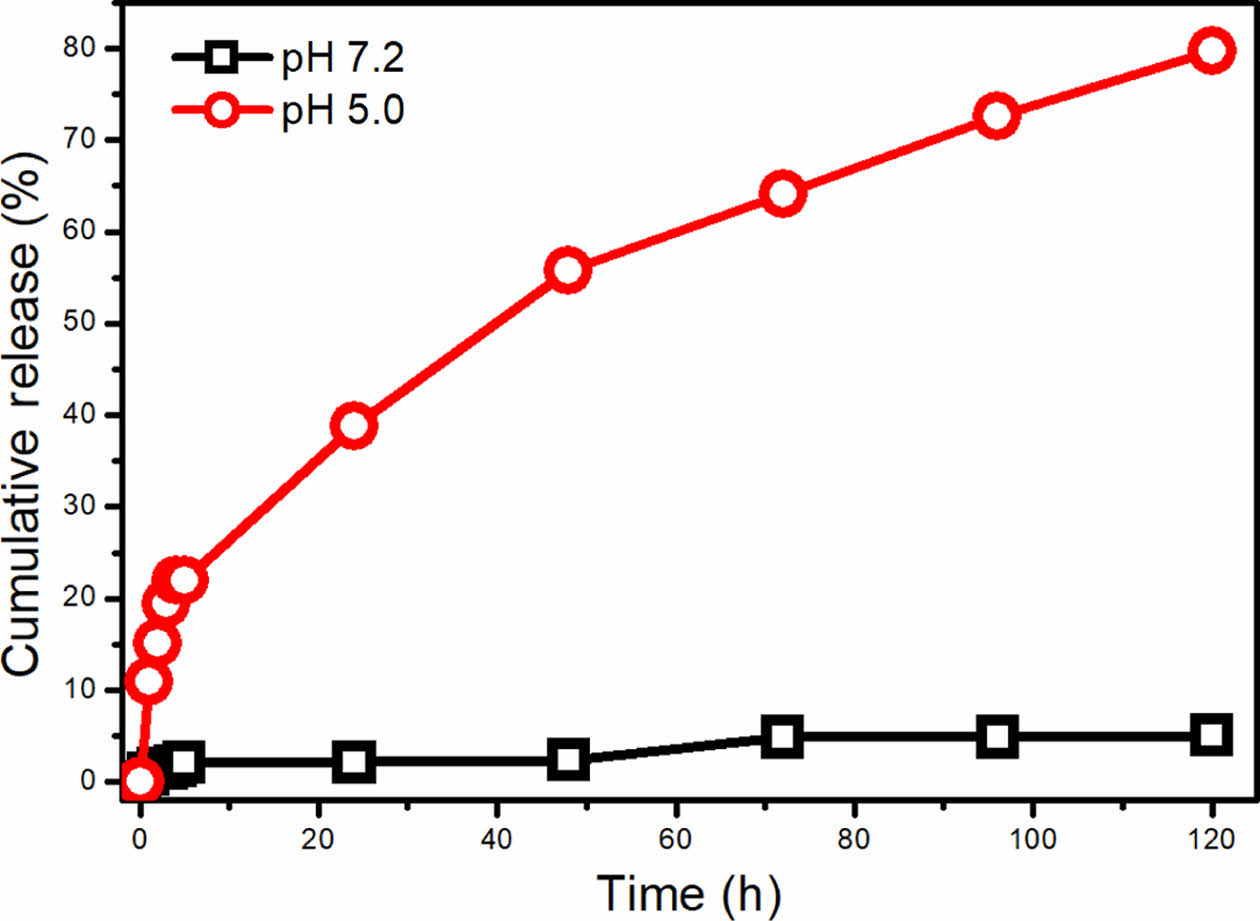
Figure 4 shows the cumulative release of CAPE from the micelle at acidic and neutral pH for 5 days. As seen, CAPE release from the micelle at physiological pH value is very low. In the first 24 hours, 2.14% of the encapsulated CAPE is released and after 5 days, only 5.04% of the drug is released. The low cumulative release can be attributed to the high hydrophobicity of the CAPE and tight packing of the micellar core. When the pH is reduced to pH 5.0, noticeably different cumulative release profile was obtained. The cumulative CAPE release reached to 38.8% after 24 hours at pH 5.0, while 79.7% cumulative release was obtained after 5 days. The remarkable increase in the release at acidic pH directly indicates the pH sensitive degradation of the PAM block in which the Schiff base (imine) groups (CH=N) are dissociated into aldehyde and amine groups.4 CAPE is released to the medium after degradation of the hydrophobic PAM block. The very low release of CAPE at neutral pH value can protect the hydrophobic cargo molecule until reaching to the lysosome or cancerous tissue.
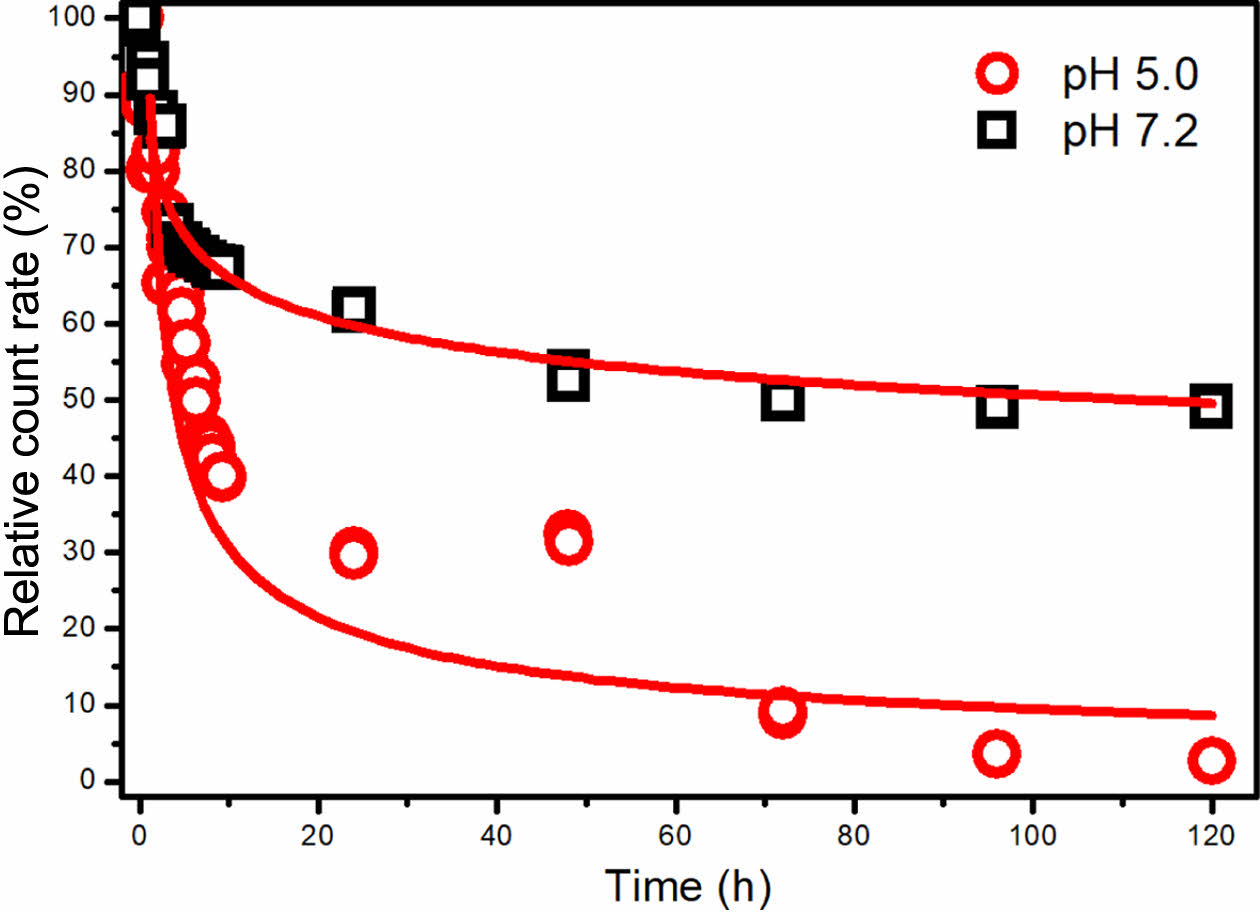
The micelles dispersed in pH 5.0 and 7.2 were analyzed with DLS to evaluate the degradation rate by investigating the derived count rates (DCR) of micellar solutions over time. The resulting DCR values were adjusted into relative count rate values by normalizing the initial and final data based on prior research findings.25,26 Figure 5 displays the time-dependent light scattering intensity (count rate) of CAPE-loaded micelles at pH 5.0 and 7.2. Generally, the count rate correlates directly with the particle size and concentration of nanoparticles in the solution.27
As demonstrated in Figure 5, micelles at pH 5.0 exhibited a sharp reduction in count rate within the initial 10 hours, followed by a slower decline, ultimately reaching 2% after 120 hours. This trend indicates a decrease in micelle size and/or quantity, suggesting their biodegradation. A comparison of this biodegradation pattern with the release profile shown in Figure 4 highlights a strong alignment between the micelle degradation times and drug release times.
Under neutral pH conditions, a relative count rate reduction of approximately 50% was observed. This reduction likely results from limited degradation at the –CH=N– bond combined with the breakdown of larger micellar aggregates into smaller entities. This disaggregation can be attributed to the stabilizing effects of salt ions present in the solution rather than significant biodegradation. In summary, the findings indicate that an acidic environment predominantly drives the rapid degradation of micelles.
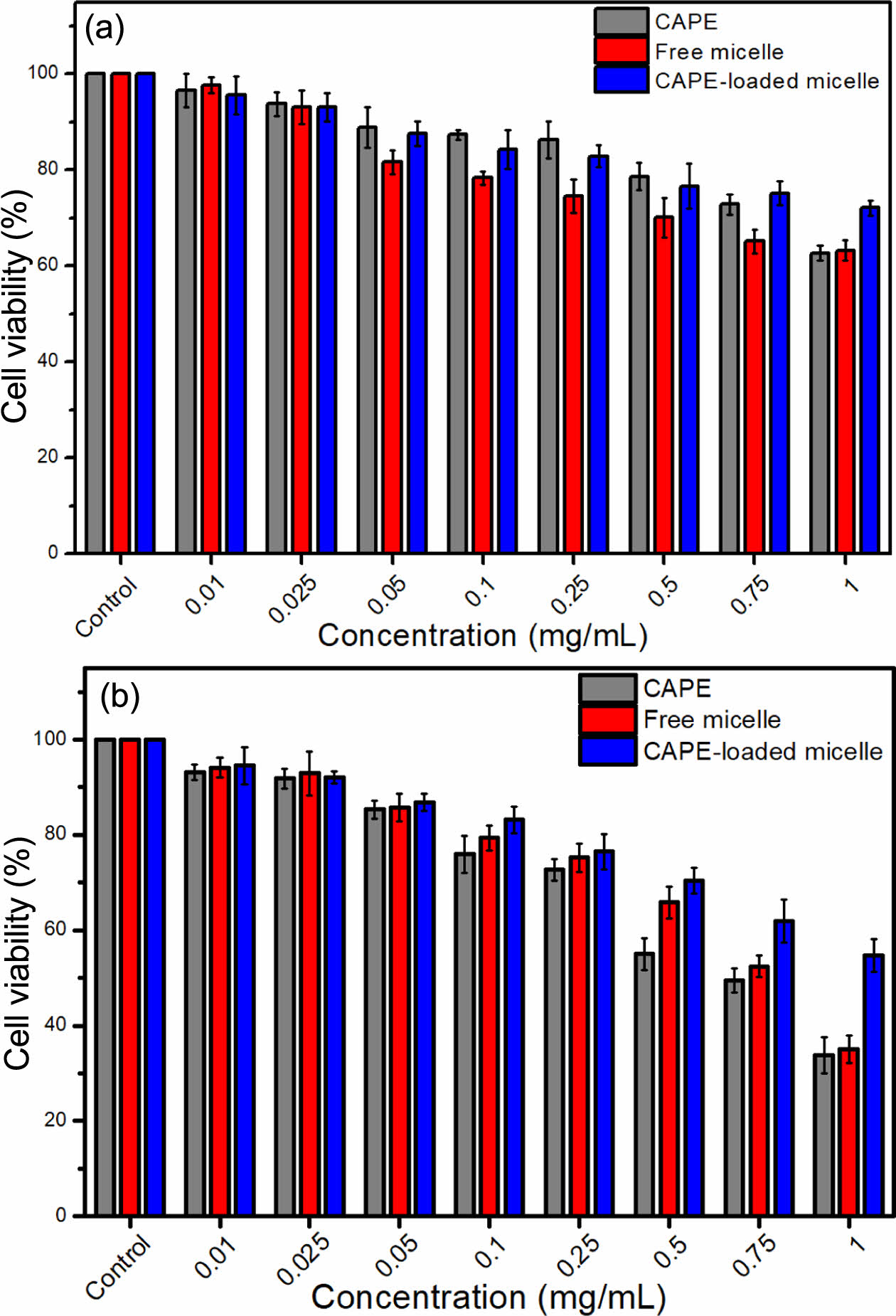
The cytotoxicity of the produced materials was investigated against HDF cells via MTT method. Figure 6 gives the viabilities of HDF cells against CAPE, free micelle and CAPE-loaded micelle after 24 (Figure 6(a)) and 48 (Figure 6(b)) hours of incubation. In MTT assay, the concentration used for CAPE-loaded micelle is the concentration of CAPE released from the micelle at the given time. After 24 h of incubation, no toxic effects were observed on the cells. However, after 48 h, CAPE-loaded micelles killed 46% of the cells, and cell viability remained above 50% (non-toxic). CAPE and free micelle killed more than 65% of the cells after 48 h. In terms of toxicity, the order was determined as CAPE > free micelles > CAPE-loaded micelles.
A critical point in the cytotoxicity study is that CAPE-loaded micelle is more biocompatible than CAPE and free micelle at almost all concentrations after 48 h of incubation. It is also worth noting that, despite having identical surface structures, the cytotoxicities of free micelles and CAPE-loaded micelles differ. This can be interpreted as CAPE and the polymer are held together tightly, which prevents the release of CAPE and consequently reduce the interaction of the polymer and the CAPE and the micelle with the cell. Therefore, the micelle is decreasing the toxicity of the CAPE and polymer to the healthy cells, which is a desired property of the micelle delivering the cargo molecule only to the anticipated cells, not to the healthy cells.
|
Figure 1 1H NMR: (a) FTIR; (b) spectra of the PAM-PEG block copolymer. |
|
Figure 2 (a) Hydrodynamic size distribution of free and CAPEloaded PAM-PEG micelles based on the intensity; (b) SEM image of CAPE-loaded PAM-PEG micelle acquired from STEM detector at 300 kX magnification. |
|
Figure 3 FTIR spectra of CAPE, free PAM-PEG micelle and CAPEloaded PAM-PEG micelle. |
|
Figure 4 Cumulative release profile of CAPE from PAM-PEG copolymer micelle at pH 5.0 and 7.2. |
|
Figure 5 Relative count rate of CAPE-loaded PAM-PEG copolymer micelle at pH 5.0 and 7.2 depending on tine. |
|
Figure 6 Viability of HDF cells against CAPE, free micelle and CAPE-loaded micelle after (a) 24; (b) 48 hours of incubation. |
This study successfully synthesized and characterized CAPE-loaded micelles using a pH sensitive PAM-PEG block copolymer. The synthesis of the PAM-PEG copolymer was confirmed through NMR and FTIR spectroscopy, demonstrating the presence of both PEG and azomethine groups. The micelles exhibited spherical morphology with an average diameter of 135.5 nm, as revealed by SEM imaging. The encapsulation efficiency and drug loading capacity of the micelles were found to be relatively low, which aligns with the known limitations of micelle-based drug delivery systems.
In vitro release studies at pH 7.2 and 5.0 indicated a controlled pH-sensitive release of CAPE, with a faster release observed at acidic pH, which is beneficial for targeting cancerous tissues. The cell viability assays using HDF cells demonstrated that CAPE-loaded micelles exhibited less cytotoxicity compared to empty micelles and free CAPE.
Overall, the study highlights the potential of PAM-PEG micelles as a viable drug delivery system for CAPE, offering controlled pH-sensitive release and passively targeted delivery capabilities. However, the low drug loading efficiency remains a challenge that needs to be addressed in future research. Another consideration is the relatively low biocompatibility of the CAPE-loaded micelles, with a viability of around 50%. Future studies could focus on optimizing the micelle structure by incorporating targeting ligands or sugar groups into the PEG chains, or by extending the PEG chain length. These modifications have the potential to improve biocompatibility, making this micelle system a promising platform for further research and development in drug delivery applications.
- 1. Ulery, B. D.; Nair, L. S.; Laurencin, C. T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B: Polym. Phys. 2011, 49, 832-864.
-

- 2. Von Burkersroda, F.; Schedl, L.; Göpferich, A. Why Degradable Polymers Undergo Surface Erosion or Bulk Erosion. Biomaterials 2002, 23, 4221-4231.
-

- 3. Xin, Y.; Yuan, J. Schiff's Base as a Stimuli-responsive Linker in Polymer Chemistry. Polym. Chem. 2012, 3, 3045-3055.
-

- 4. Baran, N. Y.; Saçak, M. Synthesis, Characterization and Molecular Weight Monitoring of a Novel Schiff Base Polymer Containing Phenol Group: Thermal Stability, Conductivity and Antimicrobial Properties. J. Molecul. Struct. 2017, 1146, 104-112.
-

- 5. Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991-1003.
-

- 6. Duan, S.; Yuan, W.; Wu, F.; Jin, T. Polyspermine Imidazole-4, 5-imine, a Chemically Dynamic and Biologically Responsive Carrier System for Intracellular Delivery of siRNA. Ang. Chem. 2012, 32, 8062-8065.
-

- 7. Dhers, S.; Vantomme, G.; Avérous, L. A Fully Bio-based Polyimine Vitrimer Derived from Fructose. Green Chem. 2019, 21, 1596-1601.
-

- 8. Jiang, X.; Ai, Y.; Han, Z.; You, Y.; Luo, H.; Cui, J.; Wei, F.; Fu, J.; He, Q.; Cheng, J. Block Copolymer-directed Synthesis of Conjugated Polyimine Nanospheres with Multichambered Mesopores. Macromol. Chem. Phys. 2020, 221, 2000061.
-

- 9. Ma, B.; Zhuang, W.; Liu, G.; Wang, Y. A Biomimetic and pH-sensitive Polymeric Micelle as Carrier for Paclitaxel Delivery. Regen. Biomater. 2018, 5, 15-24.
- 10. Sun, X.-M.; Xu, J.-X.; Tang, J.-B.; Sui, M.-H.; Shen, Y.-Q. Folate-targeted Optical and Magnetic Resonance Dualmodality PCL-b-PEG Micelles for Tumor Imaging. Chinese J. Polym. Sci. 2011, 29, 427-430.
-

- 11. Hoang Thi, T. T.; Pilkington, E. H.; Nguyen, D. H.; Lee, J. S.; Park, K. D.; Truong, N. P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298.
-

- 12. Kwon, G. S.; Kataoka, K. Block Copolymer Micelles as Long-circulating Drug Vehicles. Adv. Drug. Delivery Rev. 2012, 64, 237-245.
-

- 13. Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Delivery Rev. 2011, 63, 131-135.
-

- 14. Ozyurt, H.; Irmak, M. K.; Akyol, O.; Söğüt, S. Caffeic Acid Phenethyl Ester Changes the Indices of Oxidative Stress in Serum of Rats with Renal Ischaemia-reperfusion Injury. Cell. Biochem. Funct. 2001, 19, 259-263.
-

- 15. Ozer, M.; Parlakpinar, H.; Acet, A. Reduction of Ischemia–reperfusion Induced Myocardial Infarct Size in Rats by Caffeic Acid Phenethyl Ester (CAPE). Clin. Biochem. 2004, 37, 702-705.
-

- 16. Nah, J.-W.; Paek, Y.-W.; Jeong, Y.-I.; Kim, D.-W.; Cho, C.-S.; Kim, S.-H.; Kim, M.-Y. Clonazepam Release From Poly(DL-lactide-co-glycolide) Nanoparticles Prepared by Dialysis Method. Arch. Pharm. Res. 1998, 21, 418-422.
-

- 17. Dutta, P.; Jain, P.; Sen, P.; Trivedi, R.; Sen, P.; Dutta, J. Synthesis and Characterization of a Novel Polyazomethine Ether for NLO Application. Europ. Polym. J. 2003, 39, 1007-1011.
-

- 18. Kamaci, U. D.; Kamaci, M.; Peksel, A. Poly(azomethine-urethane) and Zeolite-based Composite: Fluorescent Biosensor for DNA Detection. Spectroch. Acta Part A: Molecul. Biomol. Spectrosc. 2019, 212, 232-239.
-

- 19. Bekri, I.; Gherras, H., Dehbi, A.; Belfedal, A. Preparation and Characterization of New Soluble and Thermally Stable Polyazomethine by Polycondensation of Thiophene-2,5-dicarboxaldehyde and Ortho-tolidine for Optoelectronics. Polym. Sci. Series B 2023, 65, 487-495.
-

- 20. Xiong, D. A.; He, Z.; An, Y.; Li, Z.; Wang, H.; Chen, X.; Shi, L. Temperature-responsive Multilayered Micelles Formed From the Complexation of PNIPAM-b-P4VP Block-copolymer and PS-b-PAA Core–shell Micelles. Polymer 2008, 49, 2548-2552.
-

- 21. Kim, S.; Shi, Y.; Kim, J. Y.; Park, K.; Cheng, J.-X. Overcoming the Barriers in Micellar Drug Delivery: Loading Efficiency, in vivo Stability, and Micelle–cell Interaction. Expert Opin. Drug Deliv. 2010, 7, 49-62.
-

- 22. Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative Evaluation of Antibacterial Activity of Caffeic Acid Phenethyl Ester and PLGA Nanoparticle Formulation by Different Methods. Nanotechnology 2015, 27, 025103.
-

- 23. Hosonuma, M.; Yoshimura, K. Association Between pH Regulation of the Tumor Microenvironment and Immunological State. Frontiers in Oncology 2023, 13, 1175563.
-

- 24. Zeng, J.; Shirihai, O. S.; Grinstaff, M. W. Modulating Lysosomal pH: a Molecular and Nanoscale Materials Design Perspective. J. Life Sci. 2020, 2, 25-37.
-

- 25. De Witte, T.-M.; Wagner, A. M.; Fratila-Apachitei L. E.; Zadpoor, A. A.; Peppas, N. A. Degradable Poly(methyl methacrylate)-co-methacrylic Acid Nanoparticles for Controlled Delivery of Growth Factors for Bone Regeneration. Tissue Eng. Part A 2020, 26, 23-24.
-

- 26. Saracoglu, P.; Dokuz, S.; Ozbek, T.; Topuzogullari, M.; Ozmen, M. M. Starch Nanogels as Promising Drug Nanocarriers in the Management of Oral Bacterial Infections. J. Drug Deliv. Sci. Technol. 2023 88, 104973.
-

- 27. Hoffmann, S.; Gorzelanny, C.; Moerschbacher, B.; Goycoolea, F. M. Physicochemical Characterization of FRET-labelled Chitosan Nanocapsules and Model Degradation Studies. Nanomaterials 2018, 8, 846.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(3): 277-284
Published online May 25, 2025
- 10.7317/pk.2025.49.3.277
- Received on Jul 9, 2024
- Revised on Nov 25, 2024
- Accepted on Dec 24, 2024
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Banu Mansuroglu
-
Department of Molecular Biology and Genetics, Faculty of Arts and Sciences, Yildiz Technical University, 34220, Türkiye
- E-mail: bmansur@yildiz.edu.tr
- ORCID:
0000-0001-8440-9118








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.