- Functionalization of Graphene Oxide with POSS Derivatives for Improved Mechanical and Thermal Properties in Epoxy Nanocomposites
Minji Kim, Garim Kim, So Youn Mun*, Kwang Youn Cho*, Man Tae Kim*,†
 , and Doojin Lee†
, and Doojin Lee† 
Department of Polymer Science and Engineering, Chonnam National University, 77 Yongbong-ro, Gwangju, 61186, Korea
*Aerospace Composites Center, Korea Institute of Ceramic Engineering and Technology, 101 Soho-ro, Jinju, 52851, Korea- 산화 그래핀의 POSS 유도체 기능화를 통한 에폭시 나노복합재의 기계적 및 열적 특성 향상
전남대학교 고분자공학과, *한국세라믹기술원 항공복합소재센터
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Graphene oxide (GO) is a carbon-based nanomaterial that serves as a reinforcement in polymer matrix, enhancing their mechanical and thermal properties. Surface treatments introduce various functional groups onto GO, facilitating strong bonding with polymer matrices and significantly improving the strength and durability of the material. In this study, the functional groups of GO are modified using aminopropylisooctyl polyhedral oligomeric silsesquioxane (POSS-amine) and a glycidyl POSS cage mixture (POSS-glycidyl). This modification aims to enhance the interfacial bonding between the filler and the matrix. GO modified with POSS-glycidyl (glycidyl-GO-POSS) exhibits improved mechanical properties and thermal conductivity compared to untreated GO, while also reducing agglomeration due to enhanced interfacial interactions with the epoxy matrix. Furthermore, the enhancement of interfacial bonding is characterized through viscoelastic analysis of both uncured and thermal-cured GO nanocomposites.
산화 그래핀(GO)은 기계적 및 열적 특성을 향상시키기 위해 고분자 매트릭스에서 강화재로 사용되는 탄소 기반 나노 소재이다. 표면 처리를 통해 GO에 다양한 기능성 작용기를 도입할 수 있으며, 이는 고분자 매트릭스와 강력한 결합을 형성하여 재료의 강도와 내구성을 크게 향상시킬 수 있다. 본 연구에서는 GO의 표면에 기능성 작용기인 아미노프로필이소옥틸 폴리헤드럴 올리고머 실세스퀴옥산(POSS-amine)과 글리시딜 POSS 케이지 혼합물(POSS-glycidyl)을 도입하여 GO와 고분자 매트릭스 간의 계면 결합을 강화하는 것을 목표로 하였다. glycidyl-GO-POSS로 수정된 산화 그래핀은 표면 처리되지 않은 GO와 비교하여 기계적 특성과 열전도성이 향상될 뿐만 아니라 에폭시 매트릭스와의 계면 결합이 강화됨에 따라 응집이 감소하는 것을 알 수 있었다. 또한, GO 나노복합재의 열 경화 전과 후의 유변 물성 비교를 통하여 POSS 처리된 GO의 계면 결합력 향상에 대한 점탄성 분석을 수행하였다.
By strategically targeting these functional groups, a robust interfacial bond can be established between the filler and the matrix. The functionalized graphene oxide (GO) has enhanced overall properties by improving the interfacial bonding strength between the polymer matrix and the modified nanofillers with polyhedral oligomeric silsesquioxane (POSS). Specifically, glycidyl-GO-POSS has improved thermal and mechanical properties due to its excellent interfacial bonding strength and exhibits uniform dispersibility.
Keywords: graphene oxide, polyhedral oligomeric silsesquioxane, rheological characterization, interfacial adhesion, nanocomposite.
This work was partly supported by Korea Research Institute for defense Technology planning and advancement (KRIT) grant funded by the Korea government (Defense Acquisition Program Administration) (KRIT-CT-23-039, Manufacturing of Asymmetric Ultra-High Temperature Ceramic Composites Leading edge with carbon fiber derived from Pitch) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) by the Ministry of Education (RS-2024-00462495).
The authors declare that there is no conflict of interest.
Epoxy resins, classified as thermosetting plastics, are widely recognized for their exceptional mechanical strength, corrosion resistance, chemical stability, and insulating properties. These characteristics have resulted in their extensive use across various industries, including adhesives, coatings, electronic packaging, automotive manufacturing, and aerospace applications.1,2 Despite their versatility, the widespread application of epoxy resins is limited by their inherent brittleness, which stems from the high crosslinking density in their structure.3
To address these limitations, recent research has explored the use of rigid, high-performance carbon-based nanofillers, such as carbon nanotubes (CNT), graphene, and graphene oxide (GO), as reinforcement agents.4-6 Among these, GO, a derivative of graphene, is particularly notable due to its superior performance characteristics and cost-effectiveness.7 Unlike graphene, GO features significant surface oxidation, which introduces abundant reactive functional groups, including hydroxyl, carboxyl, and epoxy groups.8 These functional groups not only enhance chemical reactivity but also contribute to improved thermal conductivity compared to CNT.6 However, achieving uniform dispersion of GO within a polymer matrix remains a considerable challenge. Non-uniform distribution can impede the overall performance of GO-based composites, making effective dispersion techniques essential.9-11 The functional modification of GO is crucial for addressing this issue, as it improves its dispersion within the polymer matrix and enhances interfacial interactions.12 Polymer composites significantly influence physical and chemical properties when the interfacial bonding strength between fibers and matrices is enhanced.13 In addition, uniform dispersion is essential for interfacial bonding strength, which can be analyzed using rheological methods.13-15
In recent years, the incorporation of polyhedral oligomeric silsesquioxane (POSS) in the fabrication of polymer composites has garnered significant attention.11 POSS exhibits a silicon-oxygen core, represented as (SiO1.5)n, where each silicon atom is bonded to organic groups.12,13 Its hybrid structure combines the advantages of organic components, such as flexibility, solubility, and high reactivity, with the benefits of inorganic components, including rigidity, strength, and thermal stability. In addition to these properties, POSS has been utilized as a surface modifier to enhance the dispersion of nanoparticles within the polymer matrix.12,16-19 This study focuses on the functional modification of GO using two types of POSS: POSS-amine and POSS-glycidyl. The objective is to improve the bonding strength between GO and the epoxy matrix.
The resulting epoxy composites were characterized by their mechanical and thermal properties, demonstrating significant improvements compared to neat epoxy. Enhanced dispersibility was confirmed through rheological characterization, highlighting the potential of this approach to address challenges associated with GO dispersion and interfacial bonding.
Materials. GO was purchased from Graphene All (South Korea). Aminopropylisooctyl polyhedral oligomeric silsesquioxane (POSS-amine) and a glycidyl POSS cage mixture (POSS-glycidyl) were obtained from Hybrid Plastics Inc. (AM0207 and EP0409, USA). The curing agent, which is based on a cycloaliphatic amine, was supplied by Kukdo Chemical Co. (KH-816, South Korea). The epoxy resin, derived from the diglycidyl ether of bisphenol F, was also provided by Kukdo Chemical Co. (YDF-170, South Korea). (3-aminopropyl) triethoxysilane (APS) was purchased from Daejung Chemical & Metals Co. (KH-550, South Korea).
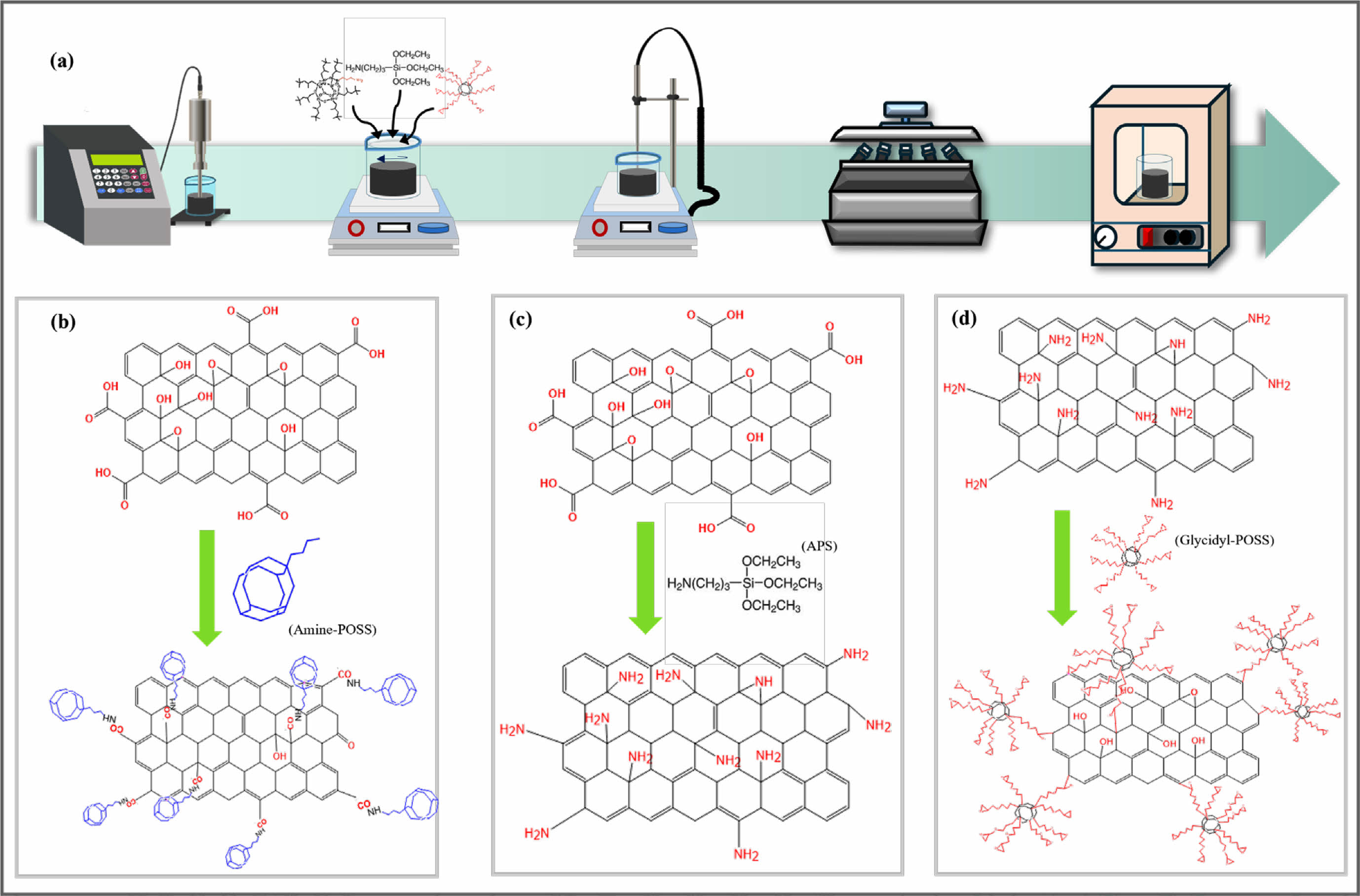
Functionalization of GO. After adding GO or GO-NH2 to the solvent, the mixture was subjected to tip sonication for 30 min in a cold bath. Subsequently, an excess of material was introduced into the solution, and magnetic stirring was performed for 12 h at 60 ℃. The mixed solution was centrifuged three times at 8000 rpm to remove unreacted materials. The final product was thoroughly dried and then heat-treated in a vacuum oven for 5 min at 200 ℃.
In case of Glycidyl-GO-POSS, firstly, the functional group of GO was modified to be an amino group (GO-NH2) by grafting APS. Subsequently, POSS-glycidyl was grafted through a substitution reaction between the amino group and POSS-glycidyl, resulting in Glycidyl-GO-POSS.
Preparation of GO/EPOXY Nanocomposites. Epoxy composites were prepared with varying concentrations of GO, amine-GO-POSS, and glycidyl-GO-POSS at 10, 15, 20, and 30 wt%. amine-GO-POSS and glycidyl-GO-POSS particles were introduced into the epoxy at these concentrations and mixed for 5 min using a planetary centrifugal mixer (ARE-310, Thinky Mixer Corporation, Japan). A KH-816 hardener, in an equivalent ratio of 20:11, was added to the mixture and mixed for an additional 6 min (2 min for mixing and 4 min for defoaming). The resulting composites were cured at 60 ℃ for 24 h.
The CNT/glycidyl-GO-POSS/EPOXY composite was prepared by maintaining a total content of CNT and glycidyl-GO-POSS at 30 wt% and adjusting only the ratio (1:29, 3:27, 5:25). First, just CNT and glycidyl-GO-POSS were mixed for 1 min using a planetary centrifugal mixer. The subsequent procedure for preparing the epoxy composites remained consistent with previous methods.
Characterization of Functionalized GO/EPOXY Composites. The synthesis of GO with POSS was verified by FTIR (Spectrum 400, PerkinElmer, USA). Field-emission scanning electron microscopy (FE-SEM, JSM-7900F, JEOL, Japan) was used to identify the morphology of the cross-section of the epoxy composites. The mechanical properties of the epoxy composites were analyzed by a universal testing machine (UTM, MCT-2150, A&D Company, Japan). The thermal properties of the epoxy composites were measured using a laser flash analyzer (LFA, LFA 467 HyperFlash, NETZSCH, Germany). The rheological properties of the uncured epoxy composites were measured using a rheometer (MCR302, Anton Paar, Austria) with a 50 mm parallel plate. The cured epoxy composites were analyzed using a Dynamic Mechanical Analyzer (DMA) (DMA850, TA, Instruments, USA) in film tension mode. Figure 1 Table 1
|
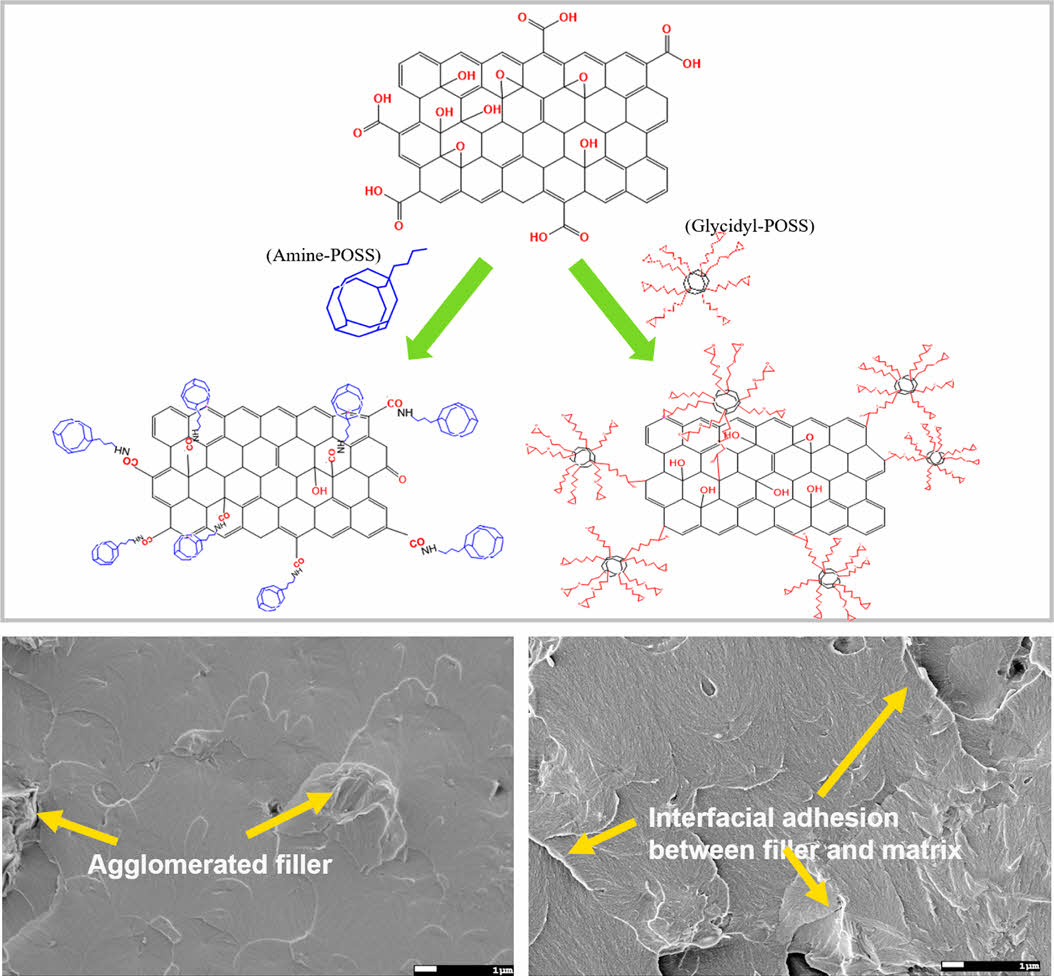
Figure 1 (a) Schematic of functionalized GO fabrication process and chemical structures of (b) GO and amine-GO-POSS; (c) GO-NH2; (d) glycidyl-GO-POSS. |
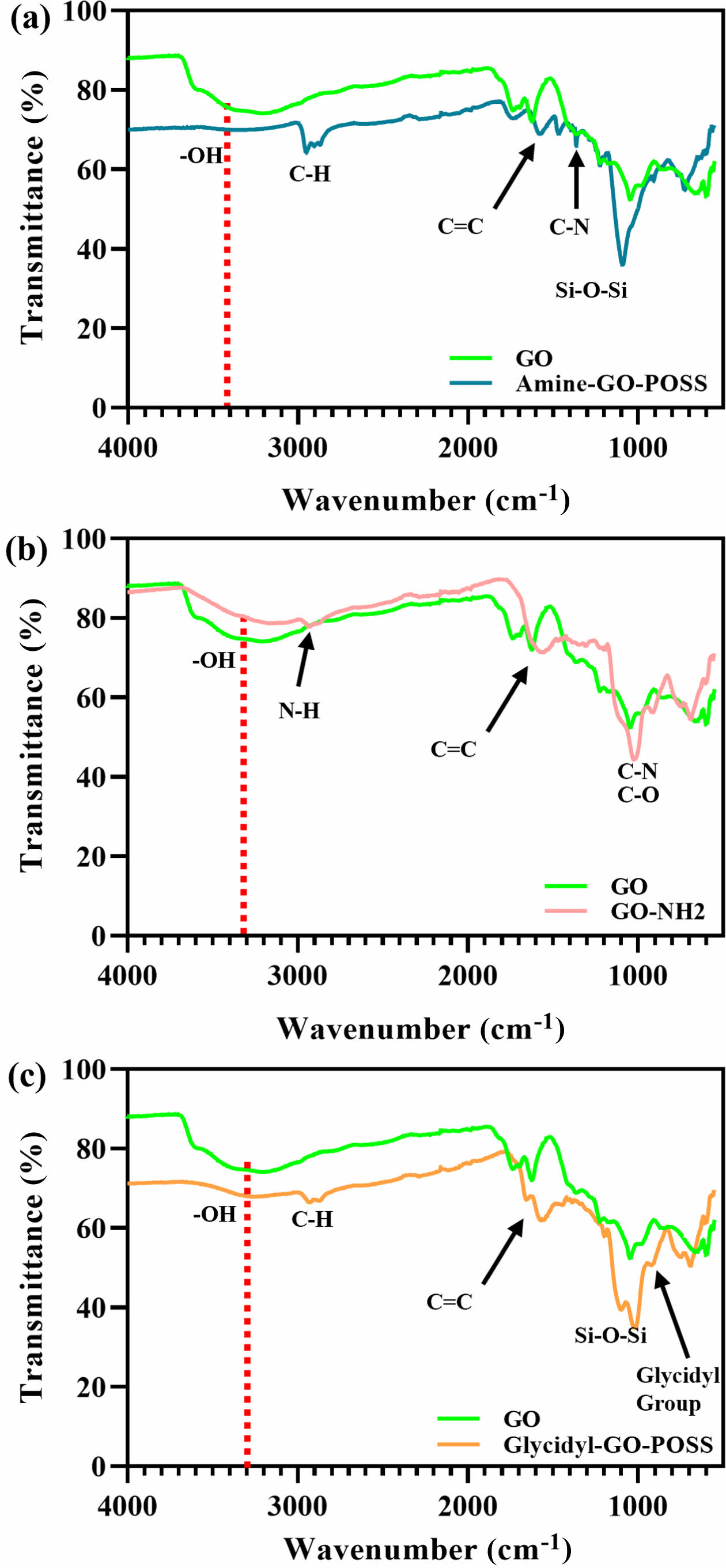
Functionalization of GO with POSS. The functionalization of POSS on the surface of GO is essential for increasing interfacial adhesion between the polymer matrix and nanoparticles. The FTIR spectra confirmed the successful introduction of POSS. Figure 2 shows the FTIR spectra of GO, amine-GO-POSS, GO-NH2, and glycidyl-GO- POSS. Figure 2(a) presents the FTIR spectra of GO and amine-GO-POSS. The broad peak at 3730 cm-1 in the GO powder corresponds to the –OH stretching vibration attributed to hydroxyl groups on the surface.11 The oxygen-containing functional groups in GO are identified by peaks at 1045, 1223, 1403, and 1773 cm-1. These peaks correspond to the stretching vibrations of C–O, C–OH, carboxyl C–O, and C=O.20 The peak at 1576 cm-1 is attributed to the stretching vibrations of the -NH2, while –C–NH2 has a distinct peak at 1357 cm-1. In particular, the strong peak at roughly 1090 cm-1 corresponds to the Si–O–Si group of POSS, indicating that POSS-amine was successfully grafted onto the GO surface.21 The grafting of APS onto GO is shown in Figure 2(b). The peak at 2885 cm-1 is associated with the stretching mode of NH2.22 Figure 2(c) shows the glycidyl-GO-POSS. The spectral area (890-950 cm-1) is distinguished by a peak at 918 cm-1, which is associated with the C–O–C (glycidyl group).20 These results demonstrate that GO can be successfully modified through covalent bonding with POSS-amine, APS, and POSS-glycidyl.
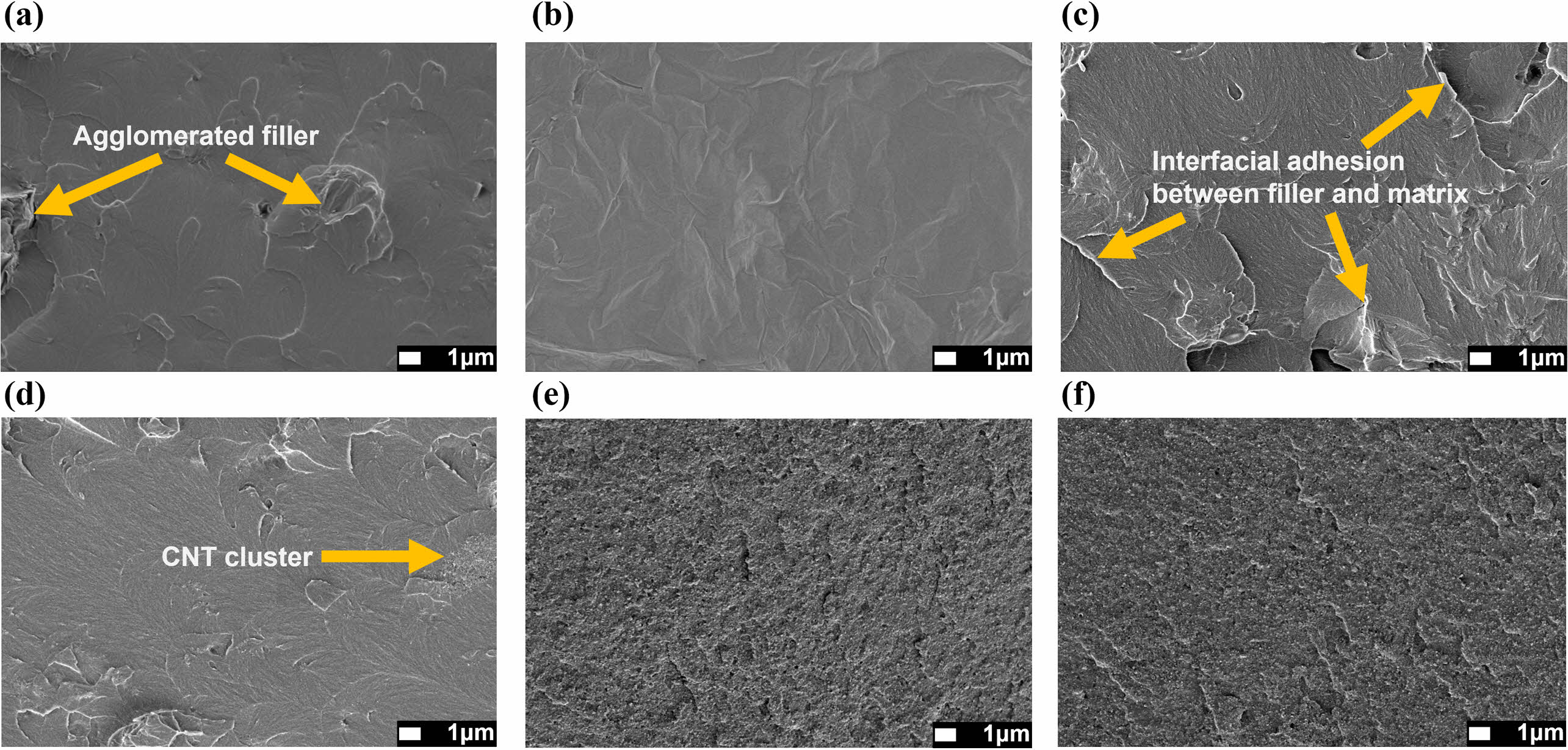
GO and CNT Dispersity in Epoxy Nanocomposites. The fracture surface was observed using FE-SEM to confirm the dispersibility of GO in the epoxy composites and to assess the interfacial bonding strength between the matrix and the filler, as shown in Figure 3. From the observations in Figure 3(a), GO demonstrates partial aggregation within the epoxy matrix. In contrast, both amine-GO-POSS and glycidyl-GO-POSS show uniform dispersion, as illustrated in Figure 3(b) and 3(c), respectively. This indicates an enhancement in dispersibility within the epoxy matrix due to the incorporation of POSS-amine and POSS-glycidyl through grafting. Notably, glycidyl-GO-POSS also displays robust interfacial adhesion with epoxy.
CNT/glycidyl-GO-POSS is shown in Figure 3(d)-(f). The CNTs are densely clustered within the matrix, forming clusters with an approximate diameter of 2.5 mm, as indicated by the arrows in Figure 3(d). As the ratio of CNTs increases, a greater number of clusters are formed in the entire area, and the cross-sectional surface becomes rougher (Figure 3(e) and 3(f)). This indicates that the dispersion of CNTs at ratios greater than 3 wt% is not optimized, resulting in agglomeration among the CNTs.
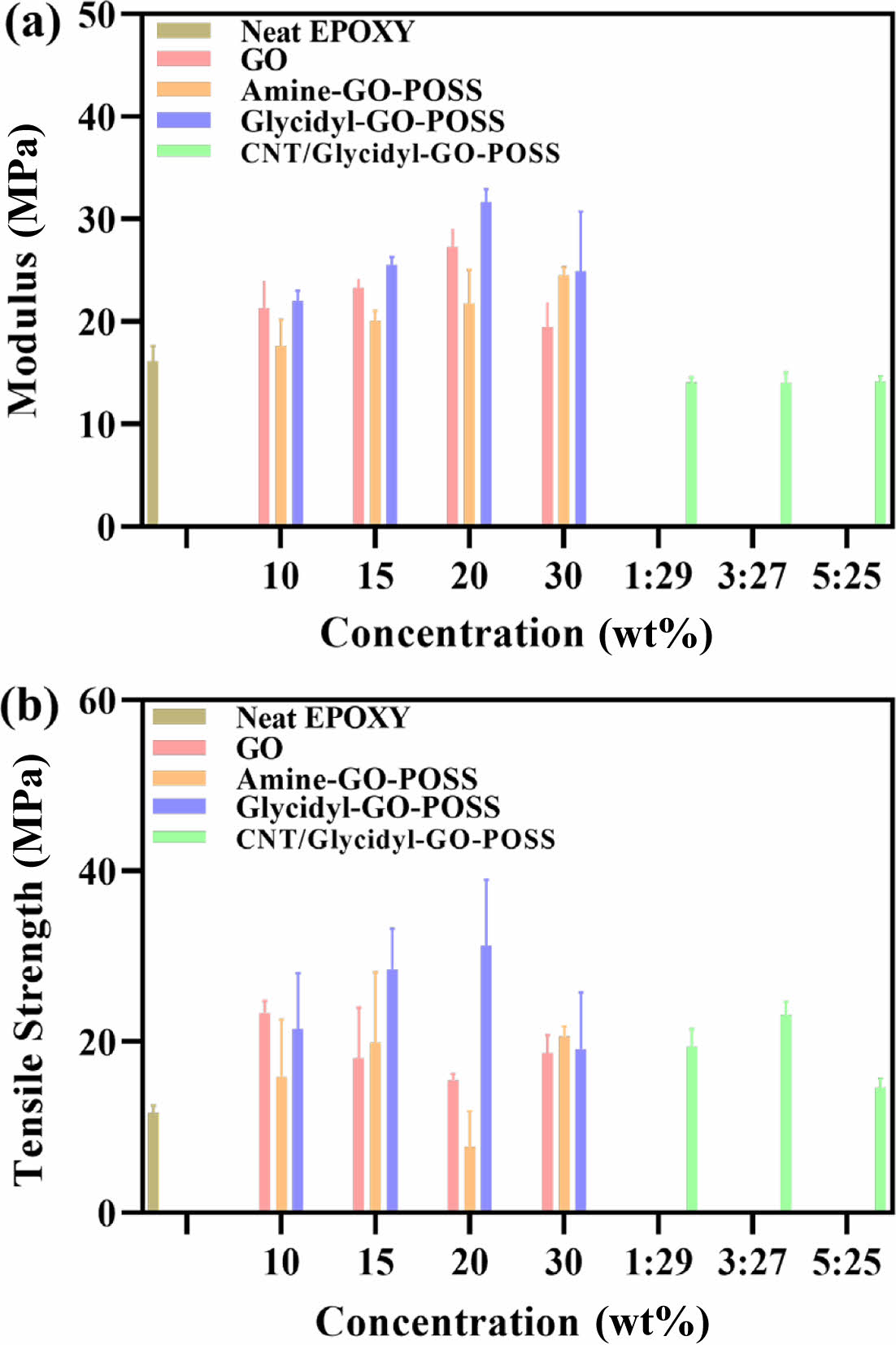
Enhancement of Mechanical Property. The representative tensile properties of neat epoxy, amine-GO-POSS/epoxy, glycidyl-GO-POSS epoxy, and CNT/glycidyl-GO-POSS are shown in Figure 3. Tensile test samples were prepared according to the ASTM D638 standard. Figure 4(a) represents Young’s modulus for each epoxy composite. The neat epoxy demonstrated Young’s modulus of 16.12 MPa. The addition of GO, amine-GO-POSS, or glycidyl-GO-POSS resulted in a significant enhancement of Young’s modulus. Notably, with a glycidyl-GO-POSS loading of 20 wt%, there was a remarkable 196.7% increase in Young’s modulus compared to the neat epoxy, reaching 31.7 MPa. This improvement is attributed to the enhanced interfacial bonding strength, facilitated by the effective binding of glycidyl groups on the glycidyl-GO-POSS surface to the epoxy matrix.21,23-24 The addition of CNTs reduced Young’s modulus, which can be attributed to the agglomeration of CNTs. The tensile strength of the epoxy composites is shown in Figure 4(b). Overall, the tensile strength increases when fillers are added. Like Young’s modulus findings, this increase was most significant at 20 wt% of glycidyl-GO-POSS, leading to a 268.7% rise.
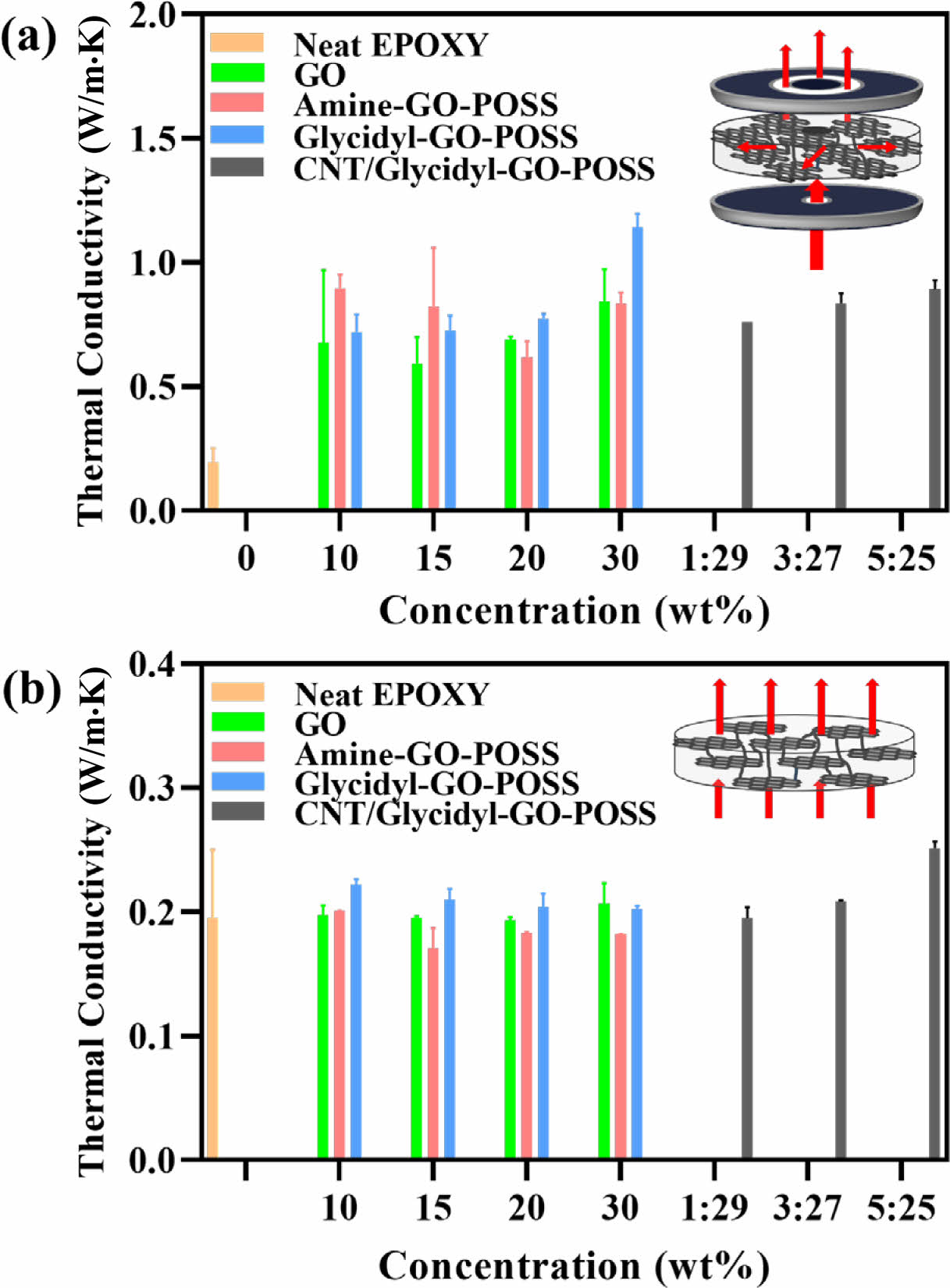
Enhancement of Thermal Conductivity. The enhancement of physical properties due to increased interfacial adhesion was remarkable, affecting not only mechanical properties but also thermal properties. The thermal conductivity of epoxy composites for each filler is illustrated in Figure 5. The thermal conductive properties of the epoxy composites were examined at 25 ℃.
The thermal conductivity in the horizontal direction is shown in Figure 5(a). The horizontal thermal conductive samples were fabricated to be 25.4 mm in diameter and 0.5 mm in thickness. The thermal conductivity of the entire epoxy composite in the in-plane direction is higher than that of neat epoxy. The GO 30 wt% composite had a thermal conductivity of 0.843 W/mK, which was 432.3% higher than that of neat epoxy. The glycidyl-GO-POSS (30 wt%) rose by 585.6%, reaching a maximum value of 1.142 W/mK. This is a 135.4% increase compared to the GO 30 wt% composite, which is due to the strong interfacial adhesion between glycidyl-GO-POSS and epoxy. The CNT/glycidyl-GO-POSS (30 wt%, 5:25) produced no significant effects on horizontal thermal conductivity; however, it exhibited the most substantial increase in vertical thermal conductivity, as shown in Figure 5(b). The vertical thermal conductive samples were prepared with a diameter of 12.7 mm and a thickness of 2 mm. The vertical thermal conductivity experienced a slight enhancement across all epoxy composites when compared to the neat epoxy. Nevertheless, the notable increase observed in the CNT/glycidyl-GO-POSS (30 wt%, 5:25) can be attributed to the synergistic effect of the bridging
phenomenon, where the CNTs function as 3D connectors between the layers of the glycidyl-GO-POSS sheet.25
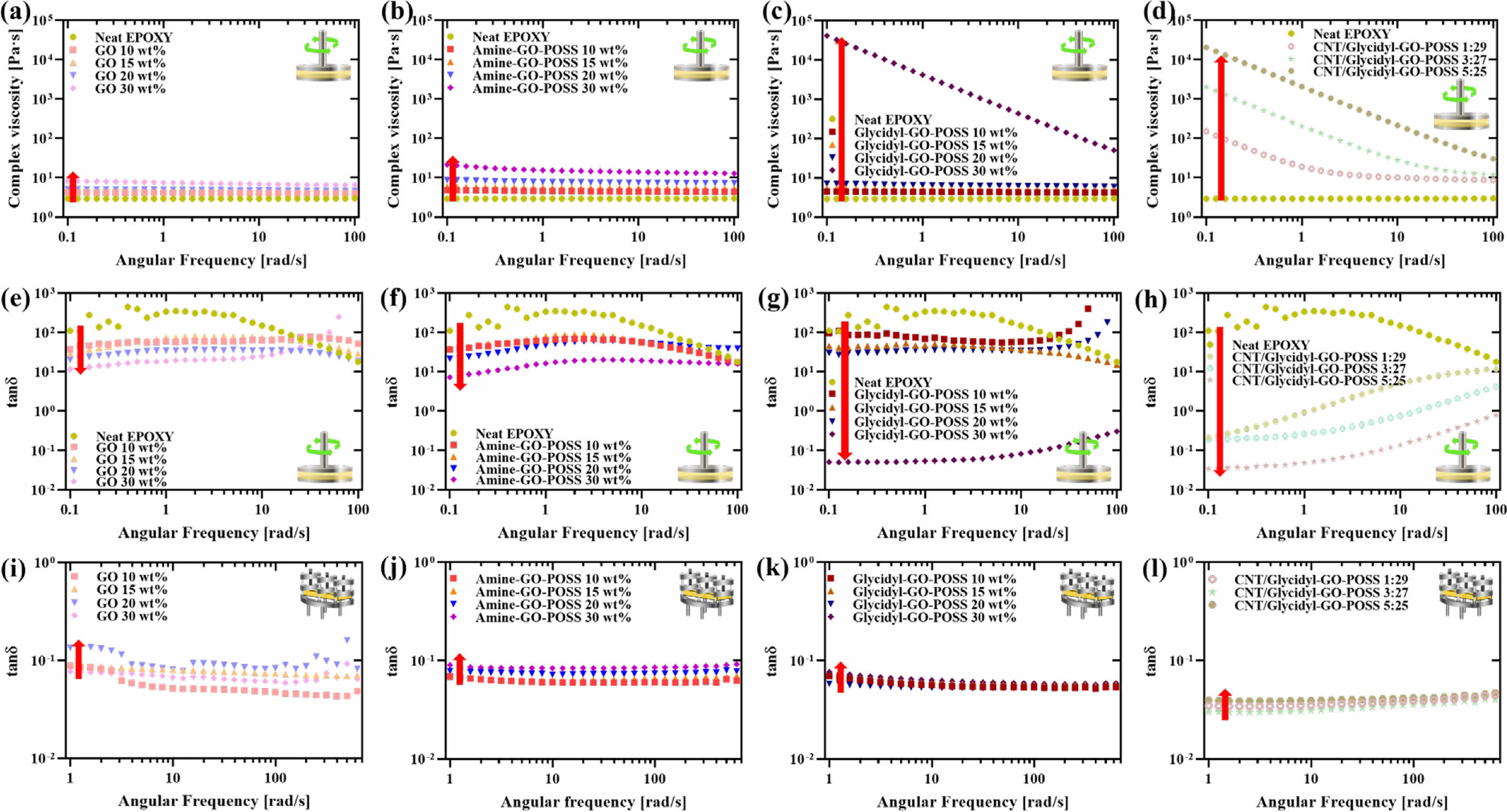
Viscoelastic Properties of Uncured and UV-cured GO/epoxy Nanocomposites. The rheological properties of the fillers in uncured epoxy composites were determined through a frequency sweep test ranging from 0.1 to 100 rad/s using a rheometer. The complex viscosities provided insights into both the dispersion of filler particles within the epoxy matrix and the viscoelastic behavior exhibited by the nanocomposites. Figure 6(a)-(d) represents the complex viscosities of the nanocomposites with varied compositions. The overall trend indicated a rise in complex viscosity with increasing filler content. Notably, amine-GO-POSS demonstrated a slight increase compared to GO, particularly evident at 30 wt%. As observed with amine-GO-POSS, glycidyl-GO-POSS also exhibited a significant increase at 30 wt%. This suggests a favorable interface bonding strength of functionalized GO and reduced agglomeration compared to untreated GO.26 Additionally, the complex viscosity of CNT/glycidyl-GO-POSS increased progressively with higher ratios of CNT. This phenomenon can be attributed to the increased bridging effect due to the additional connections within the glycidyl-GO-POSS sheets.
Figure 6(e)-(h) displays the tan δ values of uncured epoxy composites containing various filler contents. When a polymeric material is deformed, part of the energy is stored elastically, while the remainder is lost as heat due to its viscous nature.27 Tan δ, defined as the ratio of storage modulus (G') to loss modulus (G''), is known as a damping factor. As the increase in filler content, the tan δ value decreases, which is attributed to increased elasticity. At low frequency, 30 wt% of amine-GO-POSS exhibited a slight reduction in tan δ compared to GO, whereas 30 wt% of glycidyl-GO-POSS showed a notable decrease. This trend suggests that the epoxy composite solution becomes more elastic due to enhanced interfacial adhesion, especially with glycidyl-GO-POSS compared to amine-GO-POSS. The cured epoxy nanocomposites were analyzed for tan delta using dual cantilever for measuring DMA to investigate their rheological behaviors in the solid state, as shown in Figure 6(i)-(l). Interestingly, the behavior of epoxy composites after curing increases the tan δ value as the filler content rises, which contradicts the behavior observed before curing. In the case of GO, as the content increases, the rate of increase in tan δ is large, while for amine-GO-POSS and glycidyl-GO-POSS, the rate of increase in tan δ not only decreases but also shows low tan δ values. Notably, the tan δ values of both glycidyl-GO-POSS and CNT/glycidyl-GO-POSS marginally rise with increasing content. This is attributed to the stable elastic properties resulting from the strong interfacial bonding between the glycidyl-GO-POSS and the epoxy matrix. Additionally, the good damping effect within the nanocomposite, resulting from the strong interfacial affinity between modified-GO and the epoxy matrix, further contributes to this behavior.28
|
Figure 2 FTIR spectra of (a) GO, amine-GO-POSS; (b) GO-NH2; (c) glycidyl-GO-POSS. |
|
Figure 3 FE-SEM images of the fractured surface of epoxy composites containing 30 wt% of (a) GO; (b) amine-GO-POSS; (c) glycidylGO-POSS; (d) 1:29; (e) 3:27; (f) 5:25 at the ratio of CNT and glycidyl-GO-POSS. |
|
Figure 4 (a) Young’s Modulus; (b) Tensile Strength of epoxy-based nanocomposites. |
|
Figure 5 (a) In-plane thermal conductivity; (b) through-plane thermal conductivity of epoxy-based nanocomposites. |
|
Figure 6 Complex viscosity of uncured epoxy nanocomposites: (a) GO/epoxy; (b) amine-GO-POSS/epoxy; (c) glycidyl-GO-POSS/epoxy; (d) CNT/glycidyl-GO-POSS/epoxy. Tan δ value of uncured epoxy nanocomposites; (e) GO/epoxy, (f) amine-GO-POSS/epoxy; (g) glycidylGO-POSS/epoxy; (h) CNT/glycidyl-GO-POSS/epoxy. Tan δ value of UV-cured epoxy nanocomposites; (i) GO/epoxy; (j) amine-GO-POSS/ epoxy; (k) glycidyl-GO-POSS/epoxy; (l) CNT/glycidyl-GO-POSS. |
GO has various functional groups and shows promise as a nanofiller that improves mechanical and thermal properties. In this study, the impact of each functional group on the epoxy matrix composite was examined by modifying the functional groups of GO with two types of POSS (POSS-amine, POSS-glycidyl). The Young’s modulus and complex viscosity of glycidyl-GO-POSS and amine-GO-POSS were enhanced compared to GO, suggesting that the dispersibility of the filler within the epoxy matrix influences rheological properties. Particularly, glycidyl-GO-POSS exhibited the most improved dispersion characteristics, increasing Young’s modulus by up to 116.2% compared to GO. Considering these properties, the introduction of POSS-glycidyl effectively enhanced the mechanical and thermal properties due to improved interfacial bonding strength and dispersibility within the epoxy matrix. It holds great potential as a novel reinforcement for high-performance nanocomposites.
- 1. Vietri, U.; Guadagno, L.; Raimondo, M.; Vertuccio, L.; Lafdi, K. Nanofilled Epoxy Adhesive for Structural Aeronautic Materials. Compos. B. Eng. 2014, 61, 73-83.
-

- 2. Zhou, S.; Chen, Z.; Tusiime, R.; Cheng, C.; Sun, Z.; Xu, L.; Liu, Y.; Jiang, M.; Zhou, J.; Zhang, H.; Yu, M. Highly Improving the Mechanical and Thermal Properties of Epoxy Resin via Blending with Polyetherketone Cardo. Compos. Commun.2019, 13, 80-84.
-

- 3. Mishra, K.; Gajendra, P.; Raman, P. S. Enhancing the Mechanical Properties of An Epoxy Resin Using Polyhedral Oligomeric Silsesquioxane (POSS) as Nano-reinforcement. Polym. Test.2017, 62, 210-218.
-

- 4. Xu, H.; liu, Z.; Qiao, C.; Zhang, X.; Zhang, Q.; Zhang, Y.; Zheng, Y. High-performance Epoxy Composites Improved by Uniformly Dispersed and Partly Thermal Reduced Graphene Oxide Sheets. J. Appl. Polym. Sci. 2023, 140.8, e53502.
-

- 5. Pang, K.; Liu, X.; Liu, Y.; Chen, Y.; Xu, Z.; Shen, Y.; Cao, C. Highly Conductive Graphene Film with High-temperature Stability for Electromagnetic Interference Shielding. Carbon 2021, 179, 202-208.
-

- 6. Im, H.; Kim, J. H. Thermal Conductivity of a Graphene Oxide–carbon Nanotube Hybrid/epoxy Composite. Carbon 2012, 50, 5429-5440.
-

- 7. Abdullah, S. I.; Ansari, M. N. M. Mechanical Properties of Graphene Oxide (GO)/epoxy Composites. Hbrc Journal 2015, 11, 151-156.
-

- 8. Wang, M.; Ma, L.; Shi, L.; Feng, P.; Wang, X.; Zhu, Y.; Wu, G.; Song, G. Chemical Grafting of Nano-SiO2 Onto Graphene Oxide via Thiol-ene Click Chemistry and Its Effect on the Interfacial and Mechanical Properties of GO/epoxy Composites. Compos Sci. Technol.2019, 182, 107751.
-

- 9. Kim, H. W.; Yutaka, M.; Christopher W. M. Graphene/polyurethane Nanocomposites for Improved Gas Barrier and Electrical Conductivity. Chem. Mater. 2010, 22, 3441-3450.
-

- 10. Niu, R.; Gong, J.; Xu, D.; Tang, T.; Sun, Z. Y Influence of Molecular Weight of Polymer Matrix on the Structure and Rheological Properties of Graphene Oxide/polydimethylsiloxane Composites. Polymer 2014, 55, 5445-5453.
-

- 11. Kim, B. G.; Youn, B. W.; Song, Y. E.; Lee, D. J Enhanced Dispersion Stability and Interfacial Damping of POSS-functionalized Graphene Oxide in PDMS Nanocomposites. Funct. Compos. Struct. 2022, 4, 035009.
-

- 12. Zhang, M.; Yan, H.; Yuan, L.; Liu, C. Effect of Functionalized Graphene Oxide with Hyperbranched POSS Polymer on Mechanical and Dielectric Properties of Cyanate Ester Composites. RSC Adv. 2016, 6.45, 38887-38896.
-

- 13. Mun, S. Y.; Ha, J. S.; Lee, S. Y.; Ju, Y. Y.; Lim, H. M.; Lee, D. J. Prediction of Enhanced Interfacial Bonding Strength for Basalt Fiber/epoxy Composites by Micromechanical and Thermomechanical Analyses. Compos. Part A Appl. Sci. Manuf. 2021, 142, 106208.
-

- 14. Kim, B. G.; Song, Y. E.; Youn, B. W.; Lee, D. J Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-ion Battery Anode Coating. Polymers 2023, 15, 1152.
-

- 15. Ha, J. S.; Song, Y. E.; Song, N. Y.; Yun, J. S.; Lee, D. J. Designing an Interpenetrating Network of Silane-functionalized Nanocomposites for Enhanced Particle Dispersity and Interfacial Bonding Strength. Ceram. Int. 2022, 48, 1827-1835.
-

- 16. Deng, J.; Polidan, J. T.; Hottle, J. R.; Farmer-Creely, C. E.; Viers, B. D.; Esker, A. R Polyhedral Oligomeric Silsesquioxanes: a New Class of Amphiphiles at the Air/water Interface. J. Am. Chem. Soc. 2002, 124, 15194-15195.
-

- 17. Gnanasekaran, D.; Madhavan, K.; Reddy, B. S. R. Developments of Polyhedral Oligomeric Silsesquioxanes (POSS), POSS Nanocomposites and Their Applications: A Review. J. Sci. Ind. Res. 2009, 68, 437-464.
-

- 18. Jiang, D.; Xing, L.; Liu, L.; Yan, X.; Guo, J.; Zhang, X.; Zhang, Q.; Wu, Z.; Zhao, F.; Huang, Y.; Wei, S.; Guo, Z. Interfacially Reinforced Unsaturated Polyester Composites by Chemically Grafting Different Functional POSS Onto Carbon Fibers. J. Mater. Chem. A2014, 2.43, 18293-18303.
-

- 19. Jiang, D.; Xing, L.; Liu, L.; Sun, S.; Zhang, Q.; Wu, Z.; Yan, X.; Guo, J.; Huang, Y.; Guo, Z. Enhanced Mechanical Properties and Anti-hydrothermal Ageing Behaviors of Unsaturated Polyester Composites by Carbon Fibers Interfaced with POSS. Compos. Sci. Technol.2015, 117, 168-175.
-

- 20. Bai, W.; Sheng, Q.; Zheng, J. Hydrophobic Interface Controlled Electrochemical Sensing of Nitrite Based on One Step Synthesis of Polyhedral Oligomeric Silsesquioxane/reduced Graphene Oxide Nanocomposite. Talanta 2016, 150, 302-309.
-

- 21. Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous Reduction and Surface Functionalization of Graphene Oxide with POSS for Reducing Fire Hazards in Epoxy Composites. J. Mater. Chem.2012, 22.41, 22037-22043.
-

- 22. Najoul, N.; Aouida, S.; Bessais, B. Progress of Porous Silicon APTES-functionalization by FTIR Investigations. Appl. Surf. Sci. 2015, 331, 388-391.
-

- 23. Vryonis, O.; Riarh, S.; Andritsch, T.; Vaughan, A. S. Stoichiometry and Molecular Dynamics of Anhydride-cured Epoxy Resin Incorporating Octa-glycidyl POSS Co-Monomer. Polymer 2021, 213, 123312.
-

- 24. Mishra, K.; Singh, R. P. Quantitative Evaluation of the Effect of Dispersion Techniques on the Mechanical Properties of Polyhedral Oligomeric Silsesquioxane (POSS)-epoxy Nanocomposites. Polym. Compos.2018, 39, E2445-E2453.
-

- 25. Lu, H.; Zhang, J.; Luo, J.; Gong, W.; Li, C.; Li, Q.; Zhang, K.; Hu, M.; Yao, Y. Enhanced Thermal Conductivity of Free-standing 3D Hierarchical Carbon Nanotube-graphene Hybrid Paper. Compos. Part A: Appl. Sci. Manuf. 2017, 102, 1-8.
-

- 26. Kim, W.; Og, Y.; Jeon, J. Y.; Shin, B. S.; Eom, Y. H.; Chae, D. W. Combined Effect of Hybrid Carbon Fillers on the Physical and Rheological Properties of Polyvinylidene Fluoride Composites. Korea Aust. Rheol. J. 2023, 35, 137-155.
-

- 27. Kim, S. Y.; Hyun, K. Rheological and Electrical Percolation Behaviors of Polyvinyl Alcohol/silver Nanowire Suspensions Using Different Aspect Ratio Silver Nanowires. Korea Aust. Rheol. J. 2024, 36, 15-24.
-

- 28. Zhou, X.; Shin, E.; Wang, K. W.; Bakis, C. E. Interfacial Damping Characteristics of Carbon Nanotube-based Composites. Compos. Sci. Technol. 2004, 64, 2425-2437.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(3): 334-341
Published online May 25, 2025
- 10.7317/pk.2025.49.3.334
- Received on Nov 25, 2024
- Revised on Dec 20, 2024
- Accepted on Dec 23, 2024
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusion
- References
Shared
 Correspondence to
Correspondence to
- Man Tae Kim* , and Doojin Lee
-
Department of Polymer Science and Engineering, Chonnam National University, 77 Yongbong-ro, Gwangju, 61186, Korea
*Aerospace Composites Center, Korea Institute of Ceramic Engineering and Technology, 101 Soho-ro, Jinju, 52851, Korea - E-mail: ginggiscan@kicet.re.kr, dlee@chonnam.ac.kr
- ORCID:
0000-0001-7939-4812, 0000-0001-8264-4720








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.