- Scalable Fabrication of Electrochromic Films Using a Cobalt-Complexed Poly(N-vinylcarbazole) Metallopolymer
Department of Chemical Engineering, Pohang University of Science and Technology (POSTECH), Pohang, Gyeongbuk 37673, Korea
*Department of Chemistry, Kyonggi University, Suwon 16227, Korea- 코발트 배위된 폴리(N-비닐카바졸) 초분자 금속고분자를 이용한 저비용 전기변색 박막 제조법
포항공과대학교 화학공학과, *경기대학교 화학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
This study introduces a cost-effective method for fabricating electrochromic (EC) films using a cobalt-complexed poly(N-vinylcarbazole) (PVK-3-tpy-Co) supramolecular metallopolymer. The polymer, functionalized with terpyridine groups, was synthesized through a streamlined process and coordinated with cobalt ions via a simple aqueous method. The resulting EC films exhibited excellent redox stability, dynamic color modulation, and high optical contrast, as confirmed by UV-vis spectroscopy and cyclic voltammetry. This scalable approach highlights the potential of PVK-3-tpy-Co for sustainable and high-performance electrochromic applications.
이 연구에서는 코발트 배위된 폴리(N-비닐카바졸) (PVK-3-tpy-Co) 초분자 금속고분자를 이용한 경제적이고 효율적인 전기변색(EC) 박막 제조 방법을 제안한다. 테르피리딘 작용기를 도입한 이 고분자는 간소화된 합성 공정을 통해 제조되었으며, 수용액 기반의 간단한 배위 반응을 통해 코발트 이온과 결합되었다. 제조된 EC 박막은 자외선-가시광선(UV-vis) 분광법 및 순환 전압전류법(cyclic voltammetry)을 통해 분석한 결과, 우수한 산화-환원 안정성, 동적 색 변화, 높은 광학 대비를 나타내었다. 본 연구에서 제시한 확장 가능한 제조 접근법은 PVK-3-tpy-Co의 지속 가능하고 고성능 전기변색 응용 가능성을 시사한다.
This study presents a cost-effective method for fabricating electrochromic (EC) films using a cobalt-complexed poly(N-vinylcarbazole) (PVK-3-tpy-Co) metallopolymer. Functionalized with terpyridine groups and coordinated with cobalt ions via a simple aqueous method, the films exhibit excellent redox stability, dynamic color modulation, and high optical contrast. This scalable approach highlights PVK-3-tpy-Co¡'s potential for sustainable, high-performance EC applications.
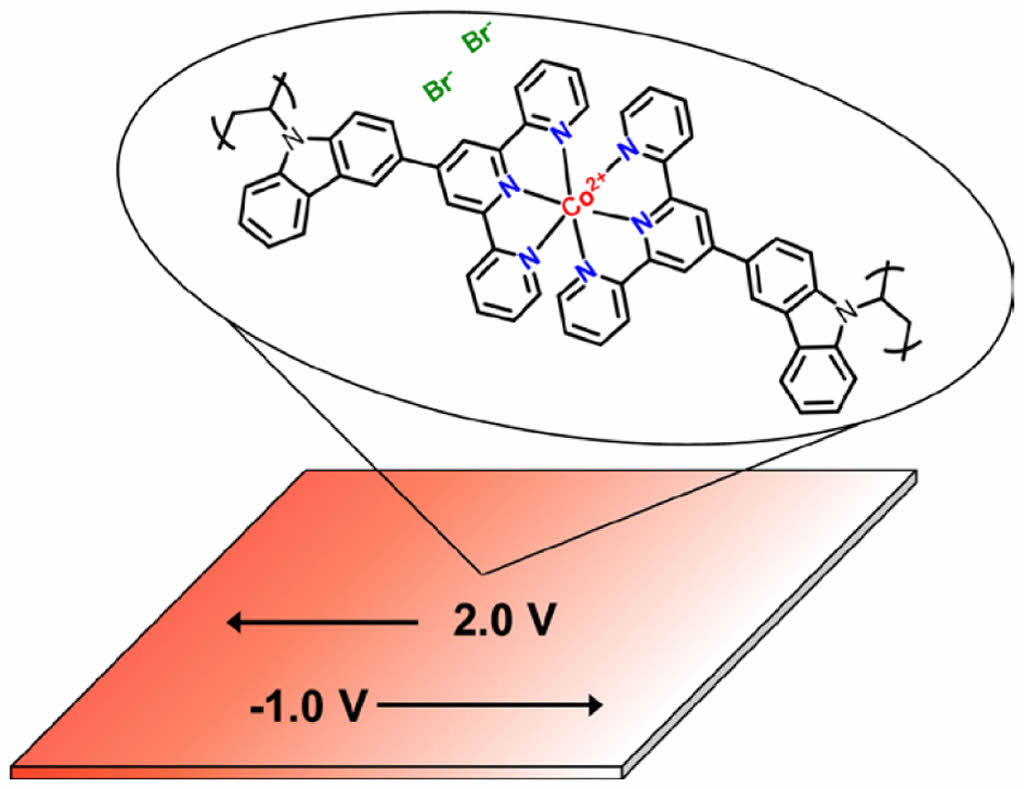
Keywords: electrochromic materials, supramolecular metallopolymers, conjugated polymers, cobalt coordination complexes, terpyridine functionalization.
This research was supported by the Technology Development Program to Solve Climate Change through the National Research Foundation of Korea (NRF) funded by ICT (RS-2024-00444389)
The authors declare that there is no conflict of interest.
Supporting Information on the synthesis and characterization of compound 3 and PVK-3-tpy, including experimental details, is available online at http://journal.polymer-korea.or.kr.
PK_2025_049_03_359_Supporting_Information.pdf (230 kb)
Supplementary Information
Electrochromic (EC) materials can reversibly modulate optical properties, such as color and transparency, via electrochemical reactions under an applied voltage.1-3 The ability of multi-color switching EC materials to cover the visible and near-infrared spectrum makes them particularly valuable for diverse industrial applications.4-6 Therefore, EC materials are extensively utilized in energy-efficient technologies, including auto-dimming windows, mirrors, and memory devices.7-10
EC materials can be broadly classified into inorganic materials (e.g., WO3, NiO), organic polymers (e.g., PEDOT), and supramolecular metallopolymers (MSPs).11-14 MSPs, in particular, have garnered significant attention for their superior electrochromic efficiency and multifunctionality, including applications in catalysis, biomedical technologies, energy storage, and photovoltaics.15-17 These unique properties are attributed to coordination bonding interactions between metal centers and polymeric matrices.18-20 Despite their potential, the synthesis of MSPs presents several challenges, including complex multi-step reactions and the difficulty of incorporating specific ligands, which impede large-scale production. Additionally, reliance on rare metals and limited recyclability poses significant sustainability concerns.20-22
In this study, we propose a novel strategy for fabricating electrochromic films utilizing MSPs based on poly(N-vinylcarbazole, PVK) polymers functionalized with terpyridine groups.23-25 Terpyridine, a versatile ligand, forms stable coordination complexes with cobalt ions, facilitating reversible oxidation state changes that result in pronounced color transitions under an applied voltage.25 This approach involves the straightforward coordination of inexpensive Co cation under aqueous conditions, achieving a simplified synthesis process.26,27 The resulting films exhibit excellent electrochromic performance, leveraging the high redox stability and cost-effectiveness of PVK while introducing enhanced versatility through metal cation complexation.
Materials and Characterization. 2-Acetylpyridine, potassium hydroxide (KOH), and ammonium hydroxide (NH4OH) were purchased from TCI (Japan). Azobisisobutyronitrile (AIBN) was obtained from Samchun Pure Chemical (Korea). All other reagents, including cobalt(II) bromide hexahydrate and solvents, were purchased from Sigma-Aldrich (USA) and used without further purification. Fluorine-doped tin oxide (FTO) glass substrates were purchased from Omniscience (Korea). Synthesis of intermediate compounds 1 and 2 was carried out following previously reported methods.28,29 Detailed procedures for the synthesis of the monomer (compound 3) and the polymer (PVK-3-tpy) are provided in the Supporting Information (SI). Proton nuclear magnetic resonance (1H NMR) spectra were recorded using a 400 MHz (Bruker BioSpin AG, Switzerland) at 298 K. UV-vis absorption spectra were measured using a Optizen Pop UV/vis spectrophotometer (Mecasys Co., Ltd., Korea) at room temperature, unless otherwise stated. Electrochemical cyclic voltammograms (CVs) of the EC films were obtained using a SP-200 instrument (Biologic SAS, France) with a three-electrode configuration: Pt wire as the counter electrode, Ag/AgCl as the reference electrode, and polymer-coated FTO glass as the working electrode. The electrolyte solution consisted of 0.1 M tetrabutylammonium hexafluorophosphate in acetonitrile (ACN).30,31
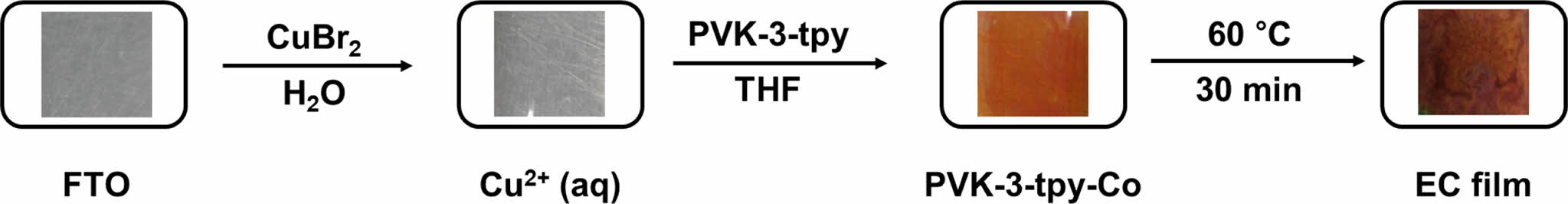
EC Film Fabrication. To fabricate the EC films, an excessive amount of Co cation solution in water was dropped onto a pre-treated FTO glass plate. Subsequently, the polymer solution (PVK-3-tpy dissolved in THF) was added dropwise. Upon addition, an immediate color change was observed, depending on the type of metal cation used. The treated plate was then heated at 60 ℃ for 30 minutes. After the film formation, the FTO plate was thoroughly washed with water and THF to remove any excess metal cations and unreacted polymer. Finally, the plate was dried under vacuum for 12 hours.
Synthesis of Monomer and Polymer. Scheme 1 outlines the synthesis pathway for the monomer and polymer. Detailed synthesis procedures and characterization are provided in SI. The monomer synthesis (compound 3) follows three key steps. First, carbazole’s nitrogen atom is alkylated with ethyl chloride to form compound 1,28 providing a precursor for further modifications. Second, the Vilsmeier-Haack reaction introduces an aldehyde group at the 3-position of the carbazole ring, yielding compound 2.29 In the final step, a condensation reaction with a terpyridine derivative produces compound 3, where the aldehyde group facilitates coupling, and the ethyl chloride group is converted into a vinyl group. This vinyl group enables radical polymerization of compound 3 using AIBN as the initiator,32,33 resulting in the polymer PVK-3-tpy. This streamlined process ensures precise functionalization and prepares both the monomer and polymer for advanced applications in metal coordination and polymeric systems.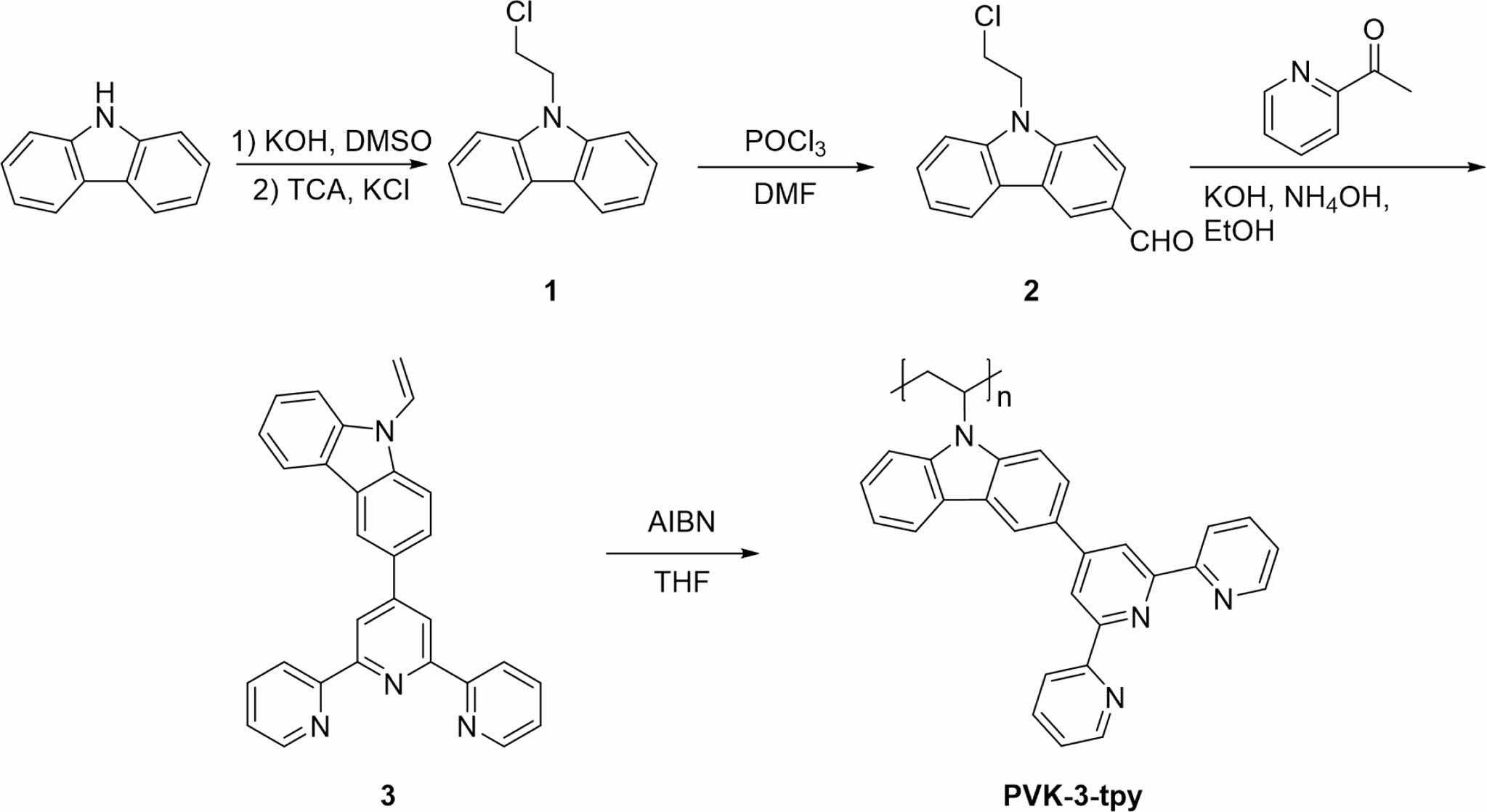
Scheme 1. Synthetic route of monomer and polymer
EC Film Fabrication with Polymer-metal Complex. A simple one-step process was developed to fabricate electrochromic (EC) films comprising polymer-metal cation complexes (Scheme 2 and Figure 1). This method involved the coordination of Co with PVK-3-tpy directly on pre-treated FTO glass. The process began with the sequential addition of a metal cation solution, followed by the polymer solution, which led to an immediate color change depending on the cation used, indicating successful complex formation. The films were then stabilized by mild heating and thoroughly washed to remove unreacted components, yielding uniform and stable EC films, denoted as PVK-3-tpy-Co. This approach provides a cost-effective and scalable strategy for EC film fabrication, eliminating the need for rare metals and high-cost processes, thereby enabling practical electrochromic applications.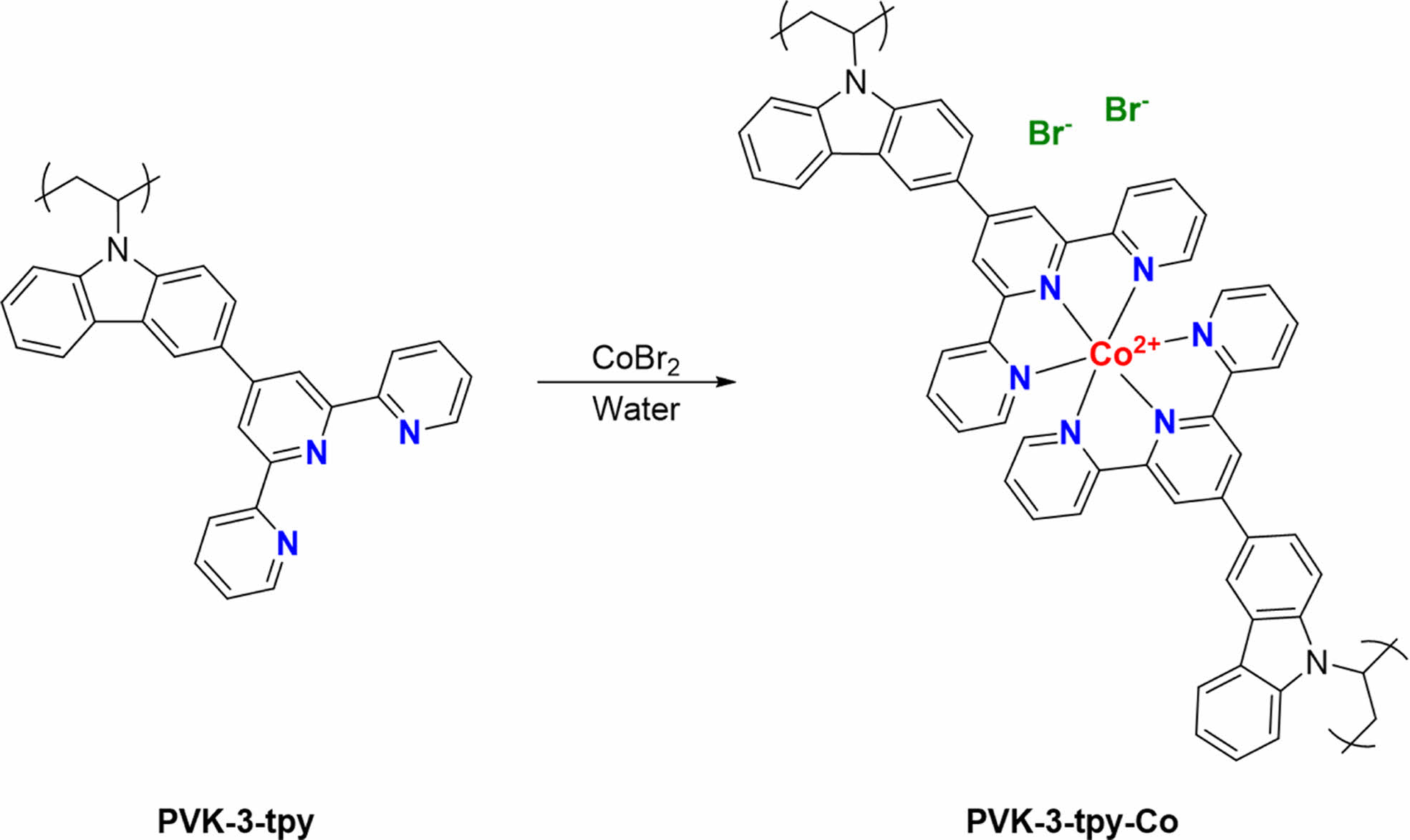
Scheme 2. Complex formation between Co2+ cations and PVK-3-tpy
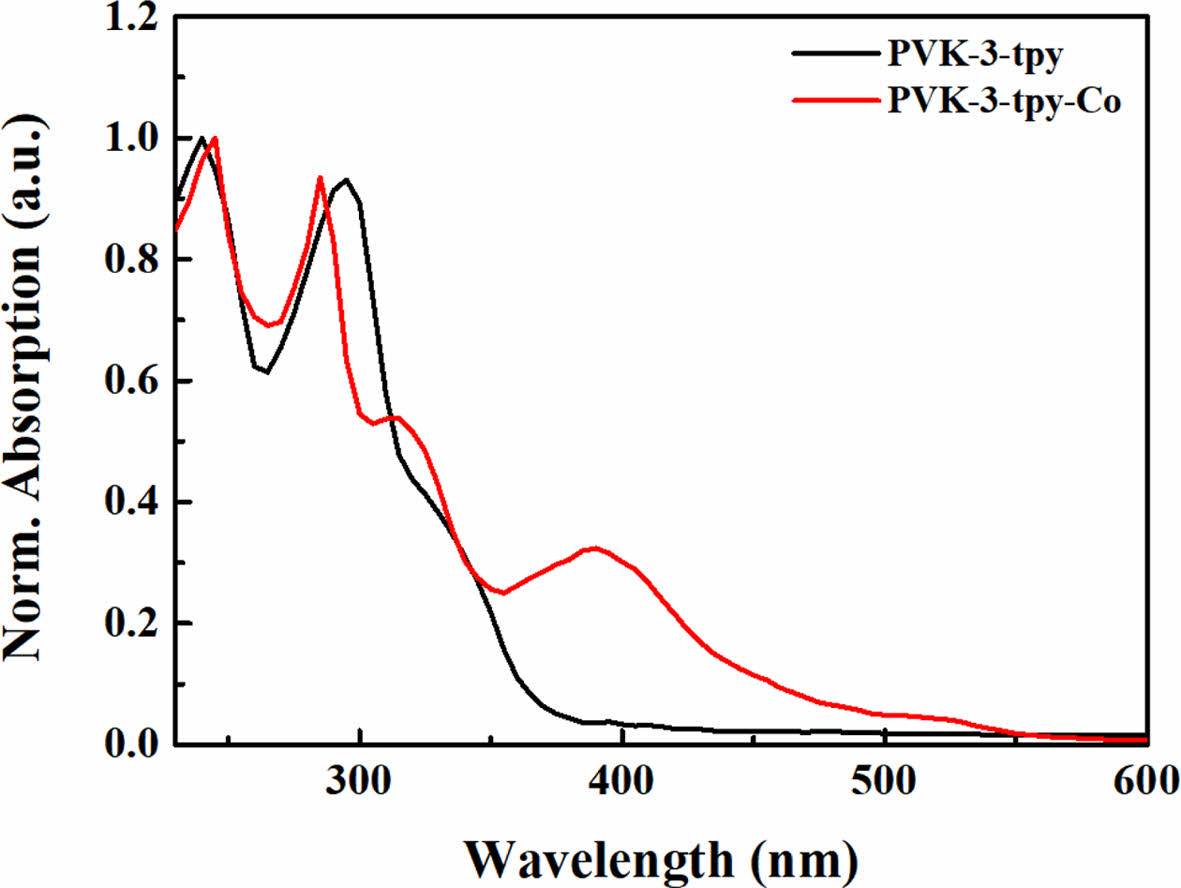
Optical Properties. The absorption spectra of the synthesized polymer in solution and its corresponding EC films are presented in Figure 2. In THF solution, PVK-3-tpy exhibits characteristic absorption peaks at 240 nm and 295 nm, attributed to the π→π* transitions of the terpyridine and carbazole moieties.34,35 The PVK-3-tpy-Co film exhibits absorption peaks at 245 nm, 285 nm, and 387 nm, with the peak at 387 nm corresponding to the Co-terpyridine complex. The low-energy absorption peak of the Co complex, observed at 387 nm, highlights the strong electronic interactions facilitated by Co2+, primarily due to its d-orbital contributions to metal-ligand charge transfer (MLCT).36,37 These spectral features emphasize the unique electronic transitions and coordination environment introduced by Co2+, demonstrating its impact on enabling the electrochromic property.
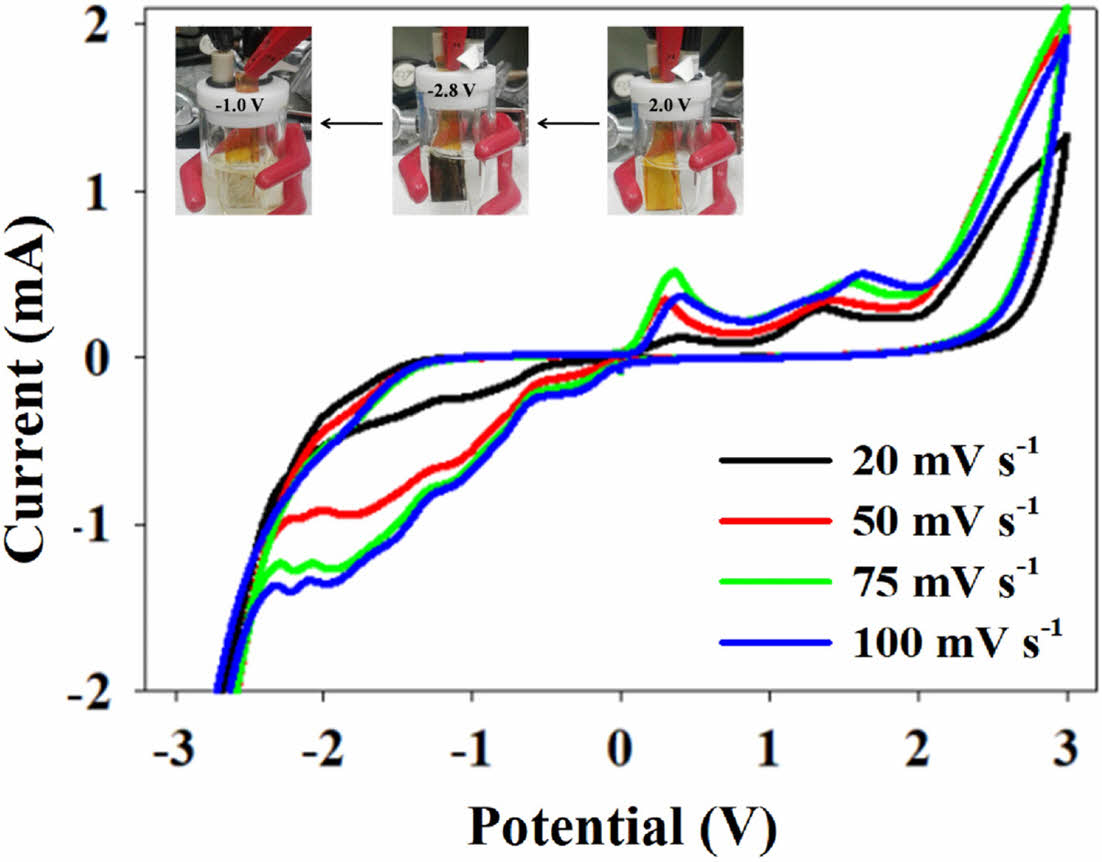
Electrochemical Properties. Cobalt exhibits multiple oxidation states, enabling significant color changes during redox transitions. As shown in Figure 3, the PVK-3-tpy-Co film displayed multiple redox potentials at -1 V, -1.75 V, +0.3 V, and +1.6 V when a potential range from -3 V to +3 V was applied. These redox transitions correspond to cobalt shifting between oxidation states (+1, +2, and +3), which its d-electron configuration and reflect changes in its electronic structure and coordination environment.38,39 The pristine orange color of the PVK-3-tpy-Co film transitioned to black at -2.7 V, bleached at -1 V, and returned to its original orange color at +1.6 V. This dynamic color modulation highlights the potential of PVK-3-tpy-Co for electrochromic applications.
CV experiments conducted at varying scan rates (25, 50, 75, 100 mV/s) revealed distinct behaviors in the oxidation and reduction processes of the PVK-3-tpy-Co film (Figure 3). The peak currents at both oxidation and reduction potentials increased proportionally with the scan rate, indicating a diffusion-controlled process.40,41 The reduction potential remained consistent across all scan rates, reflecting a reversible process with efficient reactant supply to the electrode surface. In contrast, the second oxidation potential exhibited a slight positive shift of approximately 0.2 V at 75 mV/s and 100 mV/s, after remaining stable up to 50 mV/s, suggesting that faster scan rates increase the concentration gradient near the electrode, slightly limiting reactant diffusion and necessitating marginally higher potentials to sustain the oxidation reaction.40,41 Overall, these results highlight that the electrochromic properties of PVK-3-tpy-Co are effectively maintained even at higher scan rates.
|
Figure 1 Process sequence of EC film fabrication and color changes at each stage. |
|
Figure 2 Normalized UV-Vis absorption spectra of PVK-3-tpy in THF solution and PVK-3-tpy-Co film. |
|
Figure 3 Cyclic voltammograms of PVK-3-tpy-Co recorded at different scan rates (25, 50, 75, and 100 mV s-1); inset: color switching of PVK-3-tpy-Co at various potentials. |
This study introduces a cost-effective and scalable approach for fabricating an EC film using a newly synthesized poly(N-vinylcarbazole)-based supramolecular metallopolymer (PVK-3-tpy-Co). The PVK-3-tpy polymer, functionalized with terpyridine groups, was synthesized for the first time through a streamlined process and coordinated with Co cations under aqueous conditions. This efficient one-step fabrication method successfully produced a uniform and stable EC film.
Cyclic voltammetry (CV) experiments demonstrated the stability of both oxidation and reduction processes across varying scan rates, highlighting the material’s low sensitivity to scan rate changes and its reliability under diverse conditions. These findings emphasize the electrochemical stability and consistent electrochromic performance of PVK-3-tpy-Co. By integrating a simplified synthesis route with robust redox properties, this study provides a sustainable and practical framework for developing high-performance EC materials. The results highlight the potential of PVK-3-tpy-Co for applications in smart windows, energy-efficient devices, and multifunctional technologies.
- 1. Mortimer, R. J. Electrochromic Materials. Chem. Soc. Rev. 1997, 26, 147-156.
- 2. Song, H.; Xu, M.; Yang, L.; Zhang, L.; Yan, S. Innovative Technologies for Printing, Packaging and Digital Media; Springer, 2024.
- 3. Niklasson, G. A.; Granqvist, C. G. Electrochromics for Smart Windows: Thin Films of Tungsten Oxide and Nickel Oxide, and Devices Based on These. J. Mater. Chem. 2007, 17, 127-156.
-

- 4. Nagasaki, J.; Hiroto, S.; Shinokubo, H. pi-Extended Dihydrophenazines with Three-State NIR Electrochromism Involving Large Conformational Changes. Chem.-Asian J. 2017, 12, 2311-2317.
-

- 5. Hasani, A.; Le, Q. V.; Tekalgne, M.; Guo, W.; Hong, S. H.; Choi, K. S.; Lee, T. H.; Jang, H. W.; Kim, S. Y. Tungsten Trioxide Doped with CdSe Quantum Dots for Smart Windows. ACS Appl. Mater. Interfaces 2018, 10, 43785-43791.
-

- 6. Chen, X.; Tang, P.; Yu, H.; Xu, W.; Zhao, X. Electrochemical Synthesis and Properties of Multicolor Electrochromic Triphenylamine-based Polymer Films. Macromol. Res. 2024, 32, 541-551.
-

- 7. Frolov, D. G.; Makhaeva, E. E.; Keshtov, M. L. Electrochromic Behavior of Films and «smart windows» Prototypes Based on π-conjugated and Non–conjugated Poly(pyridinium triflate)s. Synth. Met. 2019, 248, 14-19.
-

- 8. Schoot, C. J.; Ponjee, J. J.; van Dam, H. T.; van Doorn, R. A.; Bolwijn, P. T. New Electrochromic Memory Display. Appl. Phys. Lett. 1973, 23, 64-65.
-

- 9. Kim, J.; Rémond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic Conjugated Polymers for Multifunctional Smart Windows with Integrative Functionalities. Adv. Mater. Technol. 2020, 5, 1900890.
-

- 10. Hong, X.; Yang, Y.; Chen, H.; Tao, Q. Design, Fabrication and Energy-saving Evaluation of Five-layer Structure Based Transparent Heat Mirror Coatings for Windows Application. Build. Simul. 2023, 16, 2333-2342.
-

- 11. Rakibuddin, M.; Shinde, M. A.; Kim, H. Sol-gel Fabrication of NiO and NiO/WO3 Based Electrochromic Device on ITO and Flexible Substrate. Ceram. Int. 2020, 46, 8631-8639.
-

- 12. Beaujuge, P. M.; Reynolds, J. R. Color Control in π-conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268-320.
-

- 13. Higuchi, M. Electrochromic Organic–Metallic Hybrid Polymers: Fundamentals and Device Applications. Polym. J. 2009, 41, 511-520.
-

- 14. Verma, A.; Chaudhary, P.; Tripathi, R. K.; Singh, A.; Yadav, B. C. State of the Art Metallopolymer Based Functional Nanomaterial for Photodetector and Solar Cell Application. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2807-2826.
-

- 15. Wang, Y.; Astruc, D.; Abd-El-Aziz, A. S. Metallopolymers for Advanced Sustainable Applications. Chem. Soc. Rev. 2019, 48, 558-636.
-

- 16. Han, F. S.; Higuchi, M.; Kurth, D. G. Metallo-supramolecular Polymers Based on Functionalized Bis-terpyridines as Novel Electrochromic Materials. Adv. Mater. 2007, 19, 3928-3931.
-

- 17. Baumer, N.; Matern, J.; Fernandez, G. Recent Progress and Future Challenges in the Supramolecular Polymerization of Metal-containing Monomers. Chem. Sci. 2021, 12, 12248-12265.
-

- 18. Farran, R.; Jouvenot, D.; Gennaro, B.; Loiseau, F.; Chauvin, J.; Deronzier, A. Photoinduced Charge Separation Within Metallo-Supramolecular Wires Built Around a [Ru(bpy)3](2+)-Bisterpyridine Linear Entity. ACS Appl. Mater. Interfaces 2016, 8, 16136-16146.
-

- 19. Holliday, B. J.; Swager, T. M. Conducting Metallopolymers: the Roles of Molecular Architecture and Redox Matching. Chem. Commun. 2005, 23-36.
-

- 20. Sezer, S.; Köytepe, S.; Gültek, A.; Seçkin, T. Synthesis and Stimuli-Responsive Properties of Metallo-Supramolecular Phosphazene Polymers Based on Terpyridine Metal Complexes. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3389-3405.
-

- 21. Bera, M. K.; Ninomiya, Y.; Higuchi, M. Stepwise Introduction of Three Different Transition Metals in Metallo-supramolecular Polymer for Quad-color Electrochromism. Comm. Chem. 2021, 4, 56.
-

- 22. Hoogenboom, R.; Schubert, U. S. The use of (metallo-)supramolecular Initiators for Living/controlled Polymerization Techniques. Chem. Soc. Rev. 2006, 35, 622-629.
-

- 23. Hsiao, S.-H.; Lin, S.-W. Electrochemical Synthesis of Electrochromic Polycarbazole Films from N-phenyl-3,6-bis(N-carbazolyl)carbazoles. Polym. Chem. 2016, 7, 198-211.
-

- 24. Sotzing, G. A.; Reddinger, J. L.; Katritzky, A. R.; Soloducho, J.; Musgrave, R.; Reynolds, J. R.; Steel, P. J. Multiply Colored Electrochromic Carbazole-based Polymers. Chem. Mater. 1997, 9, 1578-1587.
-

- 25. Takada, K.; Ito, M.; Fukui, N.; Nishihara, H. Modulation Between Capacitor and Conductor for a Redox-active 2D Bis(terpyridine)cobalt(II) Nanosheet via Anion-exchange. Comm. Chem. 2024, 7, 186.
-

- 26. Hannewald, N.; Enke, M.; Nischang, I.; Zechel, S.; Hager, M. D.; Schubert, U. S. Mechanical Activation of Terpyridine Metal Complexes in Polymers. J. Inorg. Organomet. Polym. Mater. 2019, 30, 230-242.
-

- 27. Pahar, S.; Majee, K.; Maayan, G. A Cobalt Complex from Terpyridine-Based Peptoid as an Efficient Catalyst for Visible Light Driven Water Oxidation. Eur. J. Inorg. Chem. 2024, 27.
-

- 28. David, R. A.; Kornfield, J. A. Facile, Efficient Routes to Diverse Protected Thiols and to Their Deprotection and Addition to Create Functional Polymers by Thiol−Ene Coupling. Macromolecules 2008, 41, 1151-1161.
-

- 29. Zhang, Y.; Hokari, H.; Wada, T.; Shang, Y.; Marder, S. R.; Sasabe, H. Synthesis of N-vinylcarbazole Derivatives with Acceptor Groups. Tetrahedron Lett. 1997, 38, 8721-8722.
-

- 30. Ryu, S. U.; Ryu, D. H.; Lee, D. H.; Rehman, Z. U.; Lee, J.-C.; Lim, H.; Shin, G.; Song, C. E.; Park, T. Dithienopyran-based Narrow-bandgap Donor Polymers: Unveiling the Potential for Semitransparent Organic Solar Cells with Enhanced NIR Absorption. Chem. Eng. J. 2024, 485, 149865.
-

- 31. Rehman, Z. U.; Haris, M.; Ryu, S. U.; Jahankhan, M.; Song, C. E.; Lee, H. K.; Lee, S. K.; Shin, W. S.; Park, T.; Lee, J. C. Trifluoromethyl-Substituted Conjugated Random Terpolymers Enable High-Performance Small and Large-Area Organic Solar Cells Using Halogen-Free Solvent. Adv. Sci. 2023, 10, e2302376.
-

- 32. Kim, S.; Kwon, J. H.; Bae, Y.; Kim, J.; Park, T.; Moon, H. C. Realizing a High-performance n-type Thermogalvanic Cell by Tailoring the Thermodynamic Equilibrium. Energy Environ. Sci. 2024, 17, 8102-8110.
-

- 33. Kim, S.; Han, D.-Y.; Song, G.; Lee, J.; Park, T.; Park, S. Resilient Binder Network with Enhanced Ionic Conductivity for High-Areal-Capacity Si-based Anodes in Lithium-Ion Batteries. Chem. Eng. J. 2023, 473, 145441.
-

- 34. Kong, C.; Peng, M.; Shen, H.; Wang, Y.; Zhang, Q.; Wang, H.; Zhang, J.; Zhou, H.; Yang, J.; Wu, J.; Yupeng, T. A Novel D-A Type Terpyridine-based Carbazole Zn(II) Complex with Enhanced Two-photon Absorption and Its Bioimaging Application. Dyes Pigment. 2015, 120, 328-334.
-

- 35. Choroba, K.; Machura, B.; Erfurt, K.; Casimiro, A. R.; Cordeiro, S.; Baptista, P. V.; Fernandes, A. R. Copper(II) Complexes with 2,2':6',2''-Terpyridine Derivatives Displaying Dimeric Dichloro-mu-Bridged Crystal Structure: Biological Activities from 2D and 3D Tumor Spheroids to In Vivo Models. J. Med. Chem. 2024, 67, 5813-5836.
-

- 36. Chen, M.; Sun, D.; Li, J.; Wang, Z.; Liu, H.; Pan, L.; Chen, H.; Ma, Z. Cobalt(ii) Terpyridine Complexes: Synthesis, Characterization, Antiproliferative Activity and Molecular Docking with Proteins and DNA. New J. Chem. 2023, 47, 18785-18793.
-

- 37. Henderson, I. M.; Hayward, R. C. Kinetic Stabilities of Bis-terpyridine Complexes with Iron(ii) and Cobalt(ii) in Organic Solvent Environments. J. Mater. Chem. 2012, 22, 21366-21369.
-

- 38. Chambers, J.; Eaves, B.; Parker, D.; Claxton, R.; Ray, P. S.; Slattery, S. J. Inductive Influence of 4′-terpyridyl Substituents on Redox and Spin State Properties of Iron(II) and Cobalt(II) Bis-terpyridyl Complexes. Inorg. Chim. Acta 2006, 359, 2400-2406.
-

- 39. Araujo, C. M.; Doherty, M. D.; Konezny, S. J.; Luca, O. R.; Usyatinsky, A.; Grade, H.; Lobkovsky, E.; Soloveichik, G. L.; Crabtree, R. H.; Batista, V. S. Tuning Redox Potentials of Bis(Imino)pyridine Cobalt Complexes: An Experimental and Theoretical Study Involving Solvent and Ligand Effects. Dalton Trans. 2012, 41, 3562-3573.
-

- 40. Yamada, H.; Yoshii, K.; Asahi, M.; Chiku, M.; Kitazumi, Y. Cyclic Voltammetry Part 2: Surface Adsorption, Electric Double Layer, and Diffusion Layer. Electrochemistry 2022, 90, 102006.
-

- 41. Sandford, C.; Edwards, M. A.; Klunder, K. J.; Hickey, D. P.; Li, M.; Barman, K.; Sigman, M. S.; White, H. S.; Minteer, S. D. A Synthetic Chemist's Guide to Electroanalytical Tools for Studying Reaction Mechanisms. Chem. Sci. 2019, 10, 6404-6422.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(3): 359-364
Published online May 25, 2025
- 10.7317/pk.2025.49.3.359
- Received on Jan 13, 2025
- Revised on Jan 27, 2025
- Accepted on Jan 28, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Minjun Kim
-
*Department of Chemistry, Kyonggi University, Suwon 16227, Korea
- E-mail: minjun.kim@kyonggi.ac.kr
- ORCID:
0000-0001-6806-4674









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.