- Design, Optimization, and Evaluation of a Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Oral Delivery of Parsley n-Hexane Fraction Utilizing Coconut Oil as the Oil Phase
Edwin J. Bunggulawa, Yeong-kwan Lee*, **, and Yong-kyu Lee*, **,†

Department of Green Bioengineering, Korea National University of Transportation, Chungju 27470, Korea
*Department of Chemical & Biological Engineering, Korea National University of Transportation, Chungju 27470, Korea
**4D Convergence Technology Institute, Korea National University of Transportation, Jeungpyeong 27909, Korea- 코코넛오일을 유상상으로 활용한 파슬리 n-헥산 분획의 경구 전달용 자기나노유화약물전달시스템(SNEDDS)의 설계, 최적화 및 평가
국립한국교통대학교 녹색바이오공학과, *국립한국교통대학교 화공생물공학과, **국립한국교통대학교 4D 바이오용합소재 산업화센터
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Herbal medicines are widely recognized for their therapeutic potential, yet the poor aqueous solubility of many active compounds often limits their bioavailability. Parsley (Petroselinum crispum), a readily available plant, is rich in bioactive metabolites with medicinal properties. This study aimed to enhance the bioavailability of parsley's n-hexane fraction by formulating a self-nanoemulsifying drug delivery system (SNEDDS). Coconut oil, a natural pharmaceutical excipient, served as the oil phase. The formulation was optimized using Design-Expert® 8.0.6 software, with droplet size, polydispersity index (PDI), and zeta potential as critical parameters. The optimized SNEDDS exhibited superior nanoemulsion properties, including an ultrafine droplet size (12.15 nm), low PDI (0.178), and a stable surface charge (-21.22 mV). Stability studies confirmed excellent thermodynamic and kinetic stability. Moreover, in vitro drug release demonstrated a biphasic release profile, achieving 100% release within 420 minutes. These results confirm the successful development of a stable SNEDDS with desirable nanoemulsion properties. The formulation offers a promising strategy to improve the bioavailability of parsley metabolites, presenting a potential platform for oral delivery of herbal compounds.
한약은 치료 효과가 뛰어난 것으로 널리 알려져 있으나, 많은 유효 성분들의 낮은 수용성으로 인해 생체이용률이 제한되는 경우가 많다. 파슬리(Petroselinum crispum)는 의약적 효능을 지닌 생리활성 물질이 풍부한 식물로, 본 연구에서는 파슬리 n-헥산 분획의 생체이용률을 향상시키기 위해 자기나노유화약물전달시스템(self-nanoemulsifying drug delivery system, SNEDDS)을 개발하였다. 코코넛오일을 유상상으로 활용하였으며, Design-Expert® 8.0.6 소프트웨어를 이용하여 평균 유효 입자 크기, 분산지수(PDI), 제타 전위를 최적화 지표로 설정하여 SNEDDS 제형을 최적화하였다. 최적화된 SNEDDS는 평균 입자 크기 12.15 nm, PDI 0.178, 제타 전위 -21.22 mV로 우수한 나노에멀전 특성을 나타냈다. 열역학적 및 운동학적 안정성 시험에서도 높은 안정성을 보였으며, in vitro 방출 시험에서는 420분 이내에 100% 방출되는 이중 상 방출(biphasic release) 양상을 보였다. 본 연구를 통해 우수한 나노유화 특성을 가진 안정한 SNEDDS 제형을 성공적으로 개발하였으며, 이는 파슬리 유효 성분의 경구 전달을 위한 효과적인 플랫폼으로 활용될 수 있음을 시사한다.
A self-nanoemulsifying drug delivery system (SNEDDS) formulation enhanced the bioavailability of parsley¡¯s hydrophobic metabolites. The optimized system exhibited ultrafine droplet size, low polydispersity index (PDI), stable zeta potential, and excellent release behavior, offering a promising platform for oral delivery of herbal compounds.
Keywords: self-nanoemulsifying drug delivery system, parsley (Petroselinum crispum), coconut oil, oral delivery.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (Grant No. 2021R1A6A1A03046418). Additional support was provided by the NRF, funded by the Ministry of Science and ICT (MSIT) of the Korean government (Grant Nos. RS-2024-00405287 and 2021R1A2C2095113).
The authors declare that they have no competing interests
Modern medicine has increasingly integrated various herbal sources into therapeutic regimens,1
with notable examples including Aspirin, derived from willow bark, and Paclitaxel, originally sourced from the Pacific yew tree. This underscores the critical need for continued exploration and identification of new therapeutic herbs to address current and emerging health challenges. Herbal medicine encompasses both traditional remedies and standardized herbal extracts used for the prevention and treatment of diseases.2
According to the World Health Organization (WHO), 60% of the world’s population relies on herbal medicine, with 80% of people in developing countries depending on it for primary healthcare. Phytocompounds and their chemical analogs have led to the development of many clinically effective drugs for both chronic and acute diseases, and ongoing research continues to search for new therapeutic agents derived from medicinal plants. The growing popularity of herbal medicine is largely driven by the belief that natural products are safe, affordable, and readily available.3
Parsley (Petroselinum crispum) is a biennial aromatic herb belonging to the Apiaceae family, distinguished by its unbranched root, pinnately divided leaves, umbels, and schizocarp fruits. Parsley is rich in flavonoids, polyphenols, furanocoumarins, carotenoids, polyacetylenes, and serves as a significant source of vitamins and minerals. Traditionally, parsley has been used to treat urinary tract disorders. Recent in vitro and in vivo studies have demonstrated a range of pharmacological activities, including diuretic, antiurolithic, hypouricemic, hypolipidemic, hypoglycemic, hypotensive, antioxidant, anti-inflammatory, and antiplatelet effects.4
However, a significant challenge in developing medicinal plant-based products is the low bioavailability of plant extracts, primarily due to poor water solubility.5
To address this issue, nanocarrier-based delivery systems offer several advantages for herbal formulations, improving pharmacokinetic and pharmacodynamic properties such as solubility, bioavailability, and stability, while also protecting against toxicity.6 One such system is the self-emulsifying drug delivery system (SEDDS), designed to enhance solubility, dissolution, and oral absorption of poorly water-soluble drugs through a simple one-step process. SEDDS generates smaller droplet sizes, which increases the interfacial surface area, improving drug absorption. The self-nanoemulsifying drug delivery system (SNEDDS) further enhances absorption by forming nanoemulsions in the gastrointestinal tract under mild agitation, keeping the drug in solution and bypassing the dissolution step.7
Additional mechanisms by which SNEDDS improves bioavailability include stimulation of pancreatic and biliary secretions, lymphatic transport, prolonged gastrointestinal residence time, increased intestinal permeability, and reduced metabolism and efflux pump activity. SNEDDS also offers practical advantages, such as ease of production and enhanced stability.8
Coconut oil was selected as the oil phase for the SNEDDS formulation due to its multiple advantages in enhancing drug delivery. This medium-chain triglyceride (MCT) oil has demonstrated efficacy in improving the solubility of poorly water-soluble drugs, as evidenced by its ability to enhance the solubility of fenofibric acid in SNEDDS systems.9
The inherent antimicrobial properties of coconut oil offer potential benefits in certain drug delivery applications, particularly when synergistic effects with active pharmaceutical ingredients are desired. For instance, a study by Hosny et al.10
reported enhanced efficacy in burn wound healing when coconut oil was combined with simvastatin, leveraging the therapeutic properties of both components. Additionally, coconut oil's status as Generally Recognized as Safe (GRAS) by the FDA and its well-documented tolerability make it suitable for various routes of administration, including oral, topical, and transdermal delivery systems.11 These attributes collectively position coconut oil as an ideal component for versatile and stable SNEDDS formulations, offering potential improvements in drug solubility, absorption, and overall therapeutic efficacy.
This study aims to develop and evaluate SNEDDS for parsley (Petroselinum crispum) fraction. The optimization process focused on key parameters including droplet size, polydispersity index (PDI), and zeta potential to select the optimal formulation. The chosen SNEDDS underwent further characterization and stability assessment. This formulation aimed to evaluate the potential of SNEDDS in enhancing the solubility and absorption of parsley's bioactive components in the gastrointestinal tract, potentially improving its bioavailability and therapeutic efficacy.
Plant Material. Fresh Petroselinum crispum (parsley) herbs were sourced from a local market in Gyeonggi-Do Province, South Korea. The herbs were thoroughly cleaned and air-dried at room temperature (22 ± 2 ℃), protected from direct sunlight. The dried herbs were then pulverized using a commercial grinder and stored in airtight containers at room temperature until further use.
Ultrasound-Assisted Extraction. Ultrasound-assisted extraction (UAE) was performed using an ultrasonic bath NXPC-B unit (KODO Technical Research Co., Ltd., South Korea) with a nominal power of 200 W and operating frequency of 40 kHz. The extraction process was carried out as follows: 100 g of powdered parsley was mixed with 1000 mL of 98% ethanol (v/v) in a 2000 mL beaker. The beaker was immersed in the ultrasonic bath filled with distilled water, and the extraction was conducted for 30 minutes at room temperature. Post-extraction, the mixture was filtered through Whatman No. 1 filter paper. The filtrate was then concentrated using a rotary evaporator (N-1210 B series, Eyela, Japan) at 50 ℃ under reduced pressure to yield the crude ethanolic extract.12
Solvent-Solvent Partitioning. The crude ethanolic extract underwent fractionation using a modified solvent-solvent partitioning method.13 Briefly, a known quantity of the extract was dissolved in 250 mL of 98% ethanol (v/v) and transferred to a 500 mL separatory funnel. An equal volume of n-hexane was added, and the mixture was gently shaken for 5 minutes, allowing for periodic venting. The resulting layers were allowed to separate, and the n-hexane fraction was collected. This process was repeated with fresh n-hexane until the n-hexane layer became nearly colorless, typically requiring 3-4 repetitions. The n-hexane fractions were pooled, and both the n-hexane and residual ethanol fractions were separately concentrated using a rotary evaporator (N-1210 B series, Eyela, Japan) at 40 ℃ under reduced pressure. The resulting fractions were weighed and stored at -20 ℃ until further analysis.
Fraction Solubility Study. The solubility of the N-hexane fraction in coconut oil was determined using a modified method based on previous study.14
Various amounts of the N-hexane fraction (5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, and 70 mg) were individually added to 10 mL of virgin coconut oil in 10 mL centrifuge tubes. Each mixture was vortexed for 30 seconds using a vortex mixer followed by sonication for 30 seconds in an ultrasonic bath (KODO Technical Research Co., Ltd., South Korea) operating at 40 kHz and 200 W. The samples were then centrifuged at 3000 rpm for 5 minutes. Visual inspection was performed to detect any precipitation or phase separation. The highest amount of N-hexane fraction that resulted in a clear, homogeneous solution was recorded as the maximum solubility.
Experimental Design and Optimization of SNEDDS. The formulation of SNEDDS was optimized using a three-component mixture design. The components were: X1 (oil phase, coconut oil), X2 (surfactant, Tween 80), and X3 (co-surfactant, PEG 400). The design space was explored using a D-optimal mixture design generated by Design-Expert® software version 8.0.6 (Stat-Ease Inc., Minneapolis, MN, USA). The responses evaluated were Y1: Particle size (nm), Y2: Polydispersity Index (PDI), and Y3: Zeta potential (mV). The experimental runs were conducted in randomized order to minimize bias. The optimization process aimed to minimize particle size and PDI while maximizing the absolute value of zeta potential. The desirability function approach was used to identify the optimal formulation that satisfied all the criteria simultaneously.
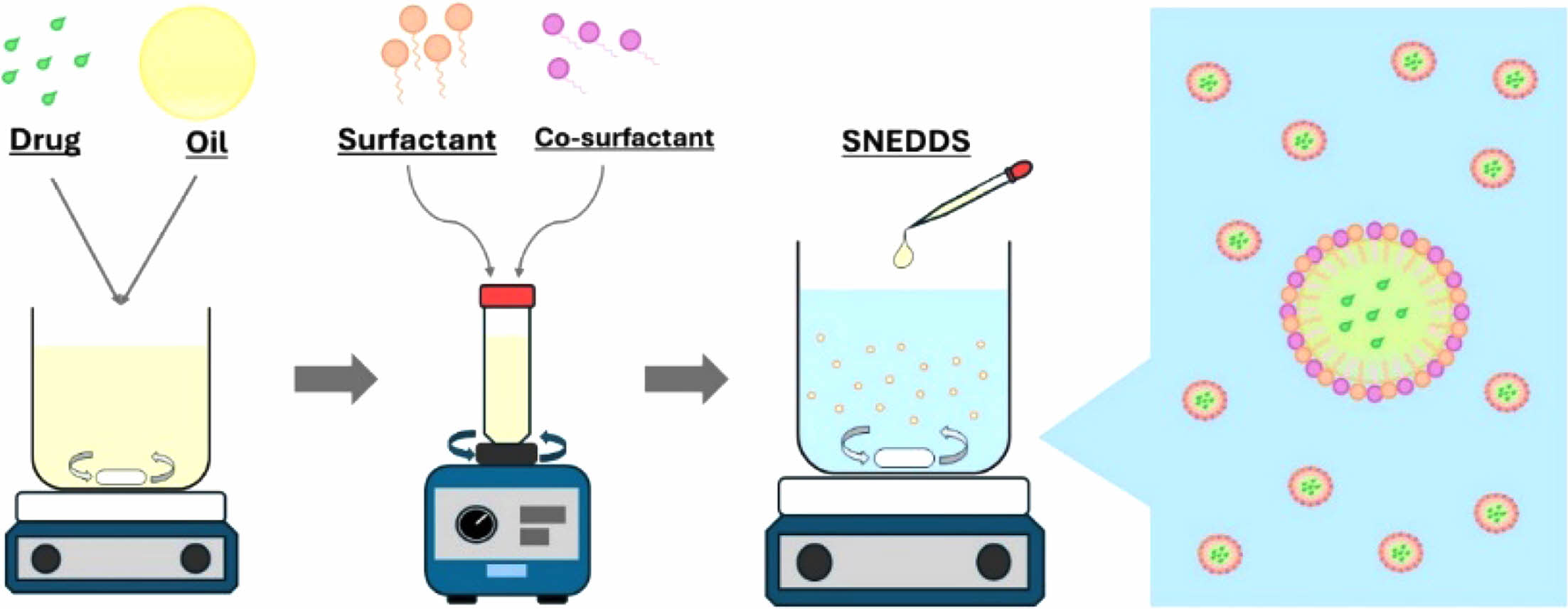
Parsley SNEDDS Formulation. The n-hexane fraction was incorporated into coconut oil in a small vial and thoroughly mixed using a vortex for 30 seconds, followed by ultrasonic treatment until complete dissolution of the fraction. The SNEDDS formulation was prepared by blending coconut oil containing the n-hexane fraction of parsley with the surfactant and co-surfactant, using a vortex for 30 seconds to ensure homogeneity. For subsequent analysis, the SNEDDS was dispersed in deionized water.
SNEDDS Evaluation. Droplet Size Analysis and Polydispersity Index: The droplet size and polydispersity index (PDI) of the nanoemulsions were determined using dynamic light scattering (DLS) (Otsuka, Japan). Prior to measurement, each SNEDDS formulation was diluted 1:10 (v/v) with deionized water.15
Zeta Potential: The zeta potential of the diluted SNEDDS formulations was measured using DLS (Otsuka, Japan). Samples were loaded into clear zeta cells, and measurements were recorded to determine the surface charge and zeta potential of the emulsion droplets.15
Clarity Test: 100 µL of SNEDDS containing parsley fraction was diluted in 10 mL of distilled water to produce a nanoemulsion. Clarity was further evaluated with a spectrophotometer at 650 nm, with distilled water as the control. Higher transmittance values indicated smaller droplet sizes, closer to the nanoscale. Subsequent quality assessments were performed on the formulation with the highest % transmittance.16
Robustness to Dilution: The robustness of selected Parsley SNEDDS formulations to dilution was evaluated by diluting them 25-, 50-, and 100-fold in deionized water. The diluted samples were stored for 24 hours and monitored for droplet size, PDI, and any physical changes, such as phase separation or drug precipitation.17-18
Emulsification Test: The emulsification time of the parsley extract SNEDDS was evaluated in three media: distilled water, artificial gastric fluid (AGF) without pepsin, and artificial intestinal fluid (AIF) without pancreatin.19
The SNEDDS parsley extract was promptly introduced into each medium, and the time required for complete emulsification was recorded. Full dissolution of the SNEDDS parsley extract in each medium indicated successful formation of the nanoemulsion.
Cryo-TEM Analysis: Cryo-TEM samples were prepared at 8 ℃ under 100% humidity using an automated vitrification device (Vitrobot Mark IV, Thermo Fisher Scientific). A 3.2 μL aliquot of the SNEDDS solution was applied to glow discharge-treated lacey carbon-coated copper grids, blotted with filter paper, and rapidly plunge-frozen in liquid ethane. The frozen samples were analyzed using a cryo-TEM (Glacios, Thermo Fisher Scientific) operated at an acceleration voltage of 200 kV.
In Vitro Drug Release Study: The drug release profile of the SNEDDS formulation was evaluated using dialysis membranes with a molecular weight cut-off (MWCO) of 2 kDa (Regenerated Cellulose, Float-A-Lyzer G2; Spectrum Labs, CA, USA). Prior to the experiment, each dialysis membrane was pre-wetted and thoroughly rinsed with deionized water to remove glycerin residues from the membrane surface and ensure optimal permeability. A 5 mL aliquot of SNEDDS solution (1 mg/mL) was transferred into the dialysis tubes, which were subsequently immersed in 200 mL of phosphate-buffered saline (PBS, pH 7, 50 mM) maintained at 37 ℃ to replicate physiological conditions. Drug release from the SNEDDS was quantified at specified intervals using a microplate reader (Synergy HTX Multimode Reader, Agilent, USA), providing precise measurements of concentration changes over time.
Stability Studies. Centrifugation Test: The Parsley SNEDDS was diluted 100-fold with deionized water and then centrifuged at 3500 rpm for 30 minutes. Phase separation was observed visually; any separation indicated differences in kinetic stability of the nanoemulsion, suggesting potential instability in the emulsion system, including phenomena such as creaming, flocculation, cracking, or coalescence.20
Heating-Cooling Cycle Test: The formulation subjected to the centrifugation test was further evaluated using a heating-cooling cycle test. This test involved six cycles at 4 ℃ and 40 ℃, with each cycle lasting at least 48 hours. Following stabilization at the test temperatures, the formulation was centrifuged at 3500 rpm for 15 minutes and visually inspected for phase separation to assess stability.21
Freeze-thaw Cycle Test: The formulation that passed the heating-cooling cycle test was evaluated with a freeze-thaw cycle test. This test involved six cycles at temperatures of -20 ℃ and 25 ℃, with each cycle lasting a minimum of 48 hours. After stabilization at ambient temperature, the formulation was centrifuged at 3500 rpm for 15 minutes and visually inspected for any phase separation to assess stability.21
Endurance Test: The formulation that passed the freeze-thaw cycle test was subjected to an endurance test. Dilutions of the formulation were prepared at 25-, 50-, and 100-fold with deionized water. Changes in % transmittance, PDI, and particle size were then measured using DLS (Otsuka, Japan) to assess formulation stability.21
Fraction Preparation and Solubility Test. Fresh parsley was sourced from a local farmer in Gyeonggi-do, South Korea. After cleaning, the herbs were shade-dried to preserve sensitive compounds and prevent oxidation. The dried herbs were then ground into a powder and extracted using 98% ethanol through ultrasound-assisted extraction, which enhances the process with ultrasonic waves.12
The extract was filtered, and the ethanol was removed using a vacuum rotary evaporator. To separate the components, the crude extract was mixed with ethanol and n-hexane in a separation funnel, allowing the compounds to separate based on their solubility. Finally, the solvents were evaporated, yielding the fractionated components.
For the formulation of the SNEDDS, which uses coconut oil as its lipid base, we evaluated the solubility of the n-hexane fraction of parsley in coconut oil. Various concentrations of the n-hexane fraction (ranging from 5 mg to 70 mg) were tested by diluting each amount in coconut oil, followed by vortexing for 30 seconds and ultrasonic treatment for another 30 seconds. The mixtures were then centrifuged at 3000 rpm for 5 minutes. Post-centrifugation, the samples were examined for phase separation or precipitation. It was observed that the maximum solubility of the n-hexane fraction in coconut oil was 50 mg, as precipitation occurred in the sample containing 55 mg of the n-hexane fraction.
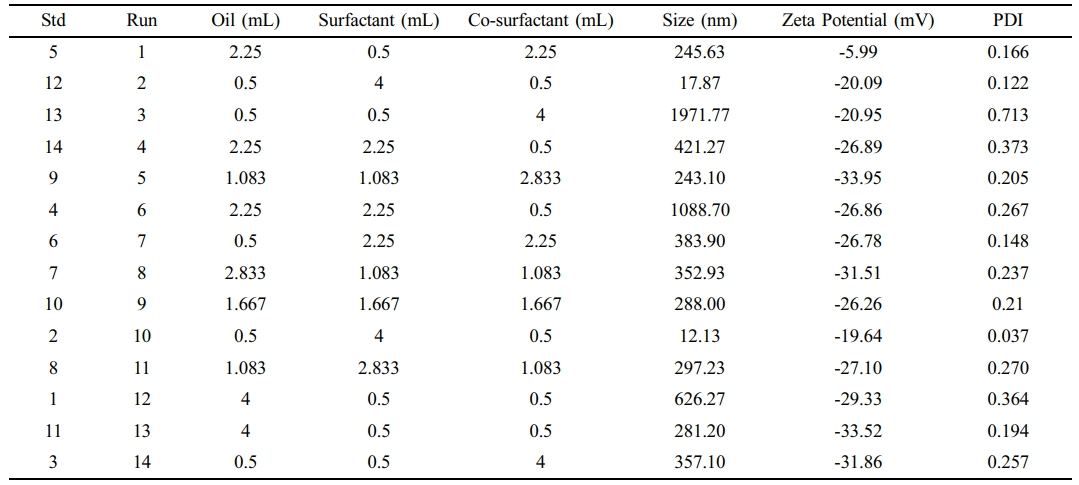
Experimental Design and Optimization of SNEDDS. For the optimization of the SNEDDS formulation, we employed the D-optimal design using Design-Expert software (version 8.0.6). Key parameters for optimization included particle size, zeta potential, and polydispersity index (PDI). The experimental formulations and their corresponding results are summarized in Table 1. Each formulation was prepared and characterized for particle size, zeta potential, and PDI using DLS. The resulting data were then input into Design-Expert for comprehensive analysis and optimization of the formulation.
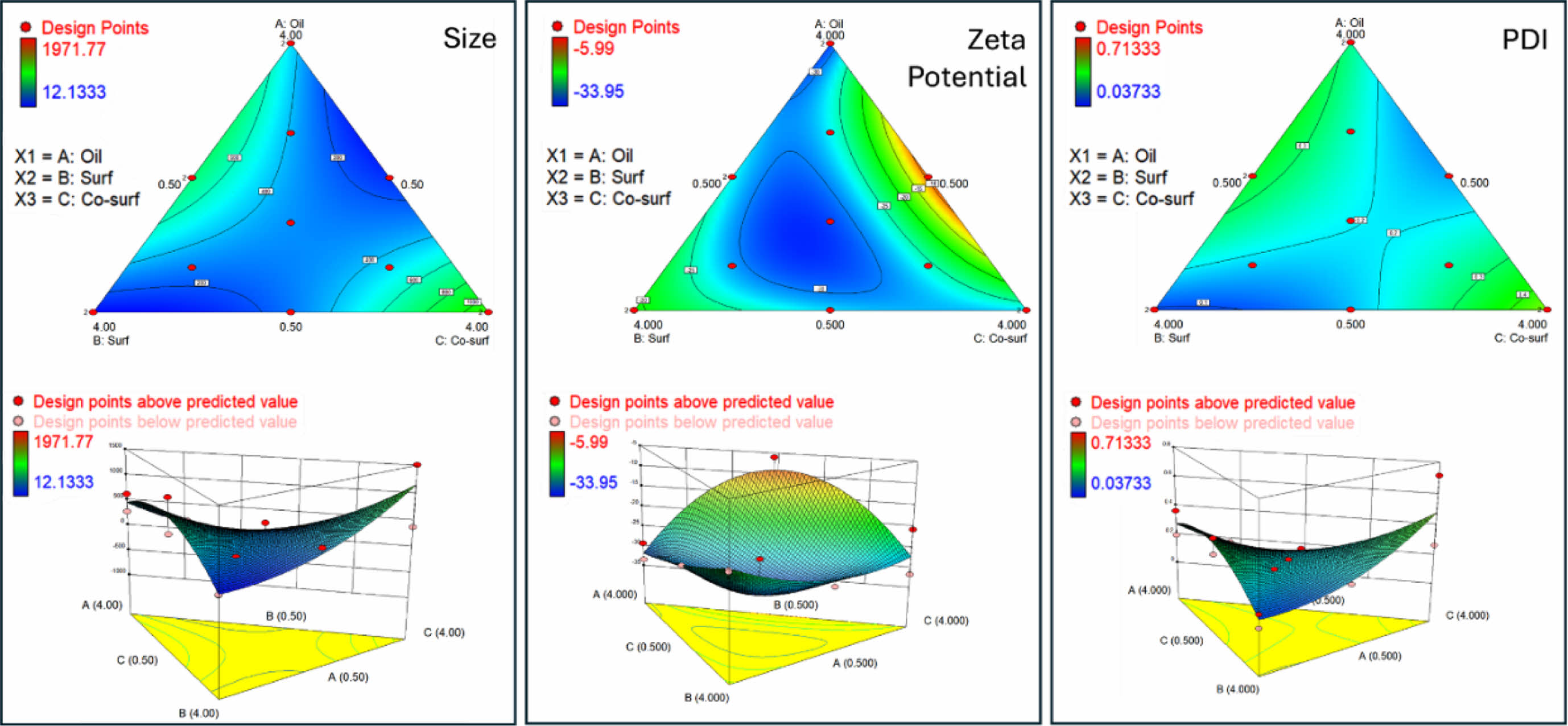
After inputting all key parameter data into the software, a ternary contour plots of oil (coconut oil), surfactant (Tween 80), and co-surfactant (PEG 400) was generated, as shown in Figure 1. The smallest particle sizes were observed in regions with high surfactant and moderate co-surfactant content, while larger sizes were found in areas with high oil and low surfactant content. This trend highlights the role of the surfactant in reducing interfacial tension and stabilizing smaller droplets. Higher surfactant concentrations enhance emulsification efficiency, resulting in smaller droplet sizes. Zeta potential and PDI followed similar trends, with high surfactant content yielding the most negative zeta potential and the lowest PDI. Conversely, high oil content resulted in higher zeta potential values and increased PDI. The ternary diagram illustrates the complex interplay between oil, surfactant, and co-surfactant in determining the system's physicochemical properties. Optimal formulations, characterized by small particle size, high stability (negative zeta potential), and low PDI, were generally achieved with high surfactant, moderate co-surfactant, and low oil content.
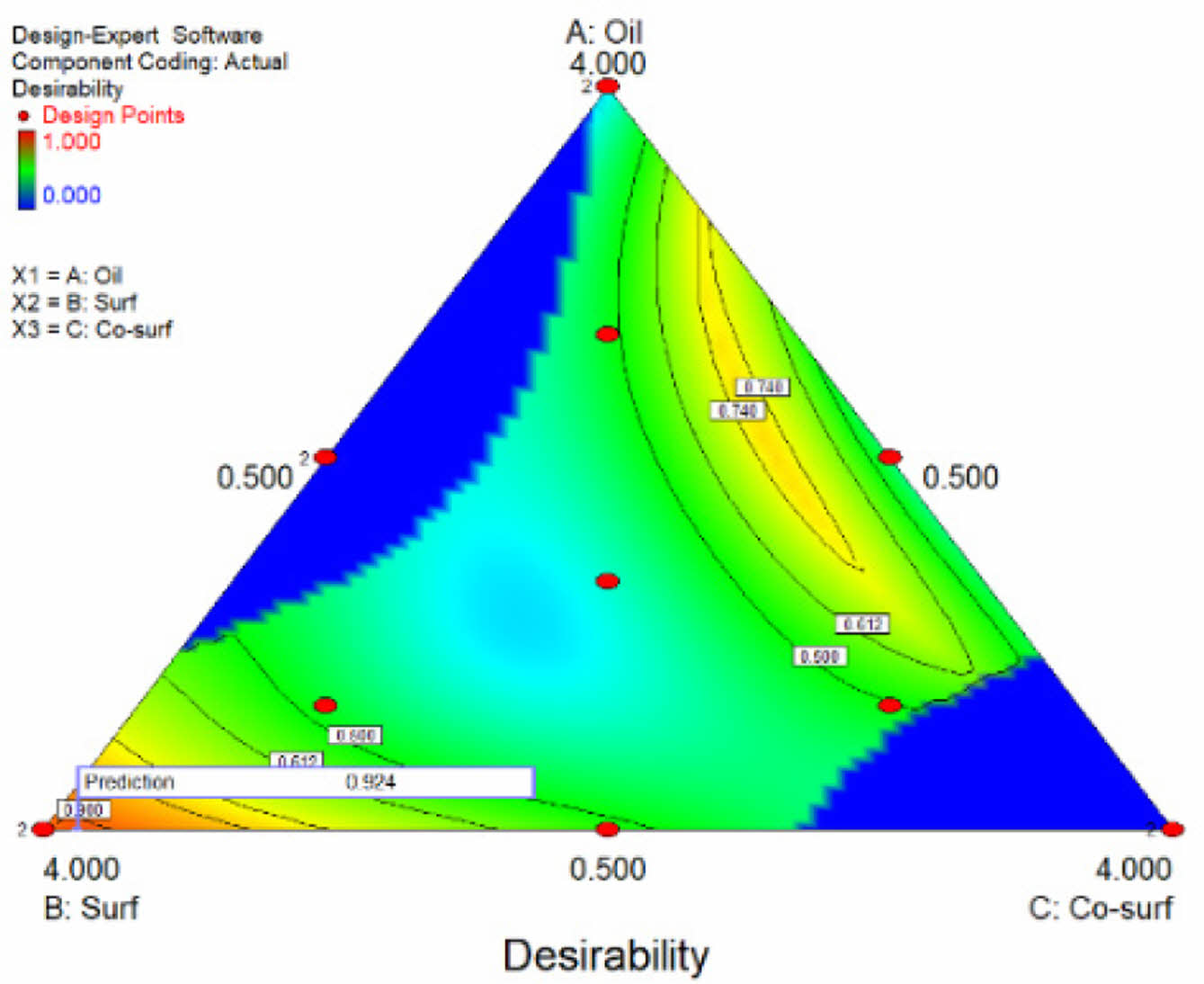
Based on the analysis of all key parameters, a desirability ternary contour plots was generated to determine the optimal SNEDDS formulation. As illustrated in Figure 2, the optimal formulation consists of moderate to high levels of surfactant, low to moderate levels of co-surfactant, and a low level of oil. Desirability increases with higher surfactant content, while excessive amounts of oil and co-surfactant reduce desirability. The software identified the optimal formulation as 0.5 mL of oil (coconut oil), 3.895 mL of surfactant (Tween 80), and 0.605 mL of co-surfactant (PEG 400), achieving a desirability value of 0.960.
The optimal formulation generated by the software suggests a high ratio of Tween 80 as the surfactant, a low ratio of coconut oil as the oil phase, and PEG 400 as the co-surfactant. This finding aligns with prior studies indicating that higher surfactant concentrations lead to smaller droplet sizes by reducing interfacial tension between the oil and water phases, facilitating the formation and stabilization of smaller droplets.22 Coconut oil, used as the oil phase in this study, increases viscosity with higher concentrations, posing challenges for droplet formation during emulsification and necessitating a higher surfactant concentration to fully encapsulate all oil droplets.23 Additionally, prior studies on nanoemulsions have shown that PEG 400, when combined with Tween 80, can synergistically stabilize emulsions, although higher concentrations of PEG may lead to increased particle size due to micelle formation.24 These findings collectively support the selected formulation parameters for achieving optimal droplet size and stability.
Parsley SNEDDS Formulation and Evaluation. The parsley SNEDDS formulation was prepared based on the optimized formula generated by Design Expert, as shown in Figure 3. Briefly, the n-hexane fraction of parsley was initially dissolved in coconut oil until fully diluted, aided by stirring and ultrasonication. A solution containing 0.5 mL of coconut oil with the n-hexane fraction of parsley was then combined with 3.895 mL of Tween 80 and 0.605 mL of PEG 400, mixed thoroughly for 30 seconds using a vortex mixer. This formulation was prepared in triplicate to validate reproducibility. For further evaluation, the parsley SNEDDS was diluted with deionized water at varying ratios.
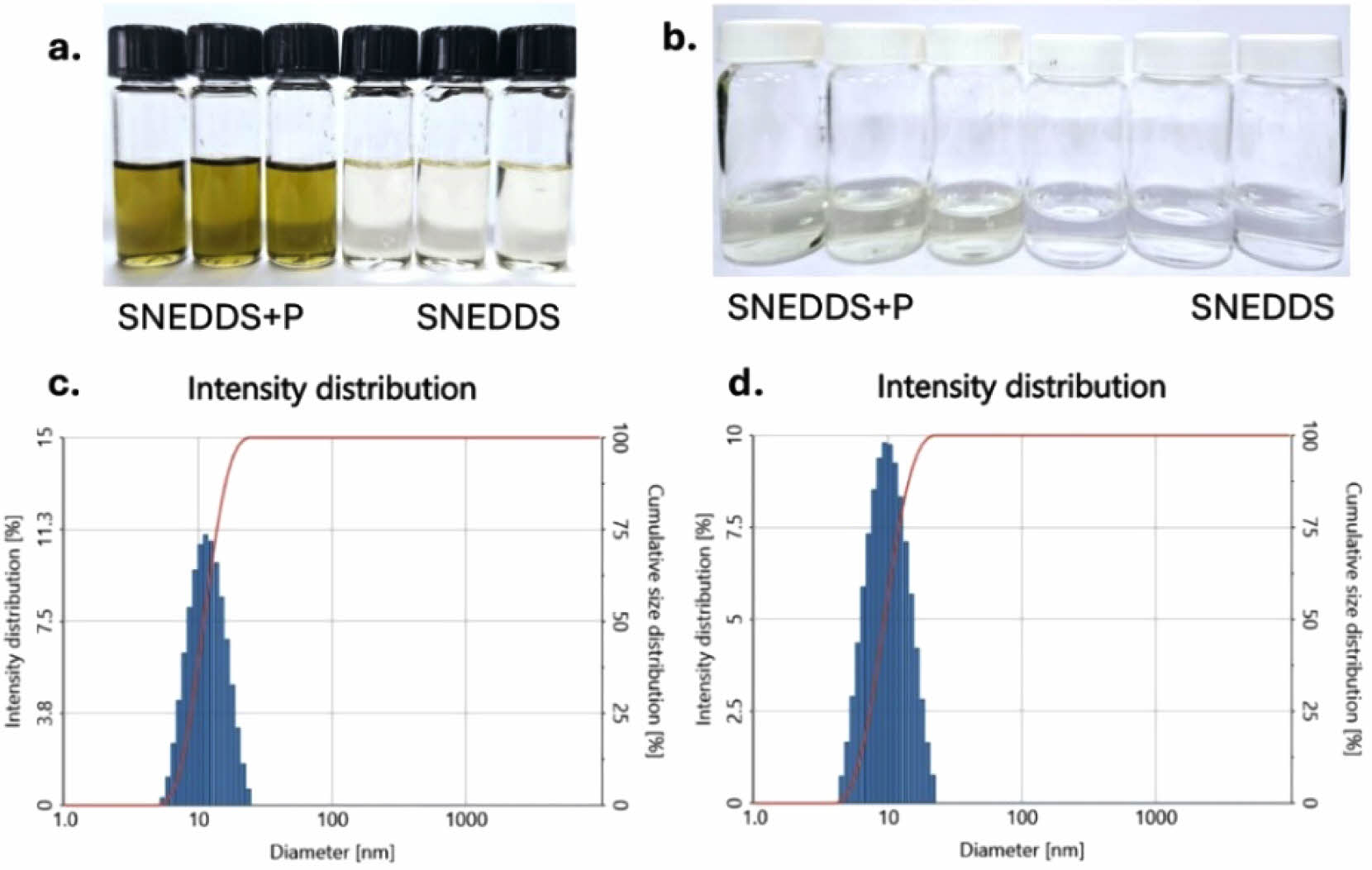
Figure 4(a) illustrates the visual appearance of the SNEDDS formulations, both with and without parsley extract. Initially, the SNEDDS formulation appears as a transparent solution, but after incorporating the n-hexane parsley fraction, it exhibits a yellow-green, transparent color. Following a 1:25 dilution in ionized water, both formulations produce well-dispersed nanoemulsions. The control SNEDDS remains a clear, transparent solution, while the parsley-enriched SNEDDS maintains a slightly yellow, transparent hue, as shown in Figure 4(b).
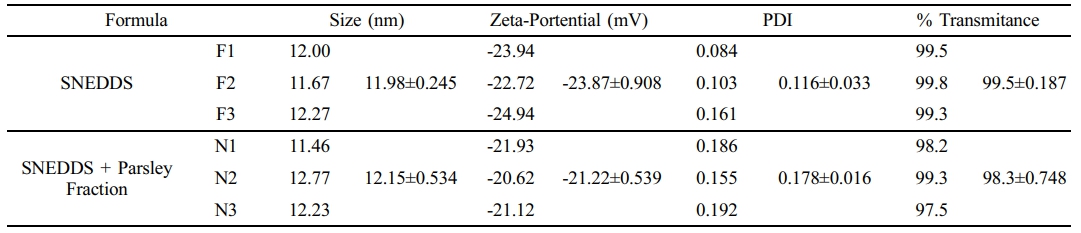
Nanoemulsion characterization data, including particle size, zeta potential, PDI, and percent transmittance, are summarized in Table 2. DLS was employed to measure particle size, zeta potential, and PDI following dilution in deionized water. The particle sizes of both SNEDDS formulations, with and without the parsley fraction, were comparable. As shown in Figure 4(c) and 4(d), the particle size increased slightly upon parsley fraction loading, from 11,98 nm for the control SNEDDS to 12,15 nm for parsley-loaded SNEDDS. This trend was also observed in zeta potential and PDI values. Specifically, the zeta potential shifted from -23.87 mV to -21.22 mV upon loading the parsley fraction, indicating a slight increase. Both formulations demonstrated good dispersibility, with PDI values of 0.116 for control SNEDDS and 0.178 for parsley-enriched SNEDDS. For clarity assessment, the percent transmittance of each formulation was measured at 650 nm using a microplate reader. The control SNEDDS exhibited 99.5% transmittance, while the parsley SNEDDS showed 98.3% transmittance, supporting the presence of small, well-dispersed particles, consistent with the DLS particle size findings.
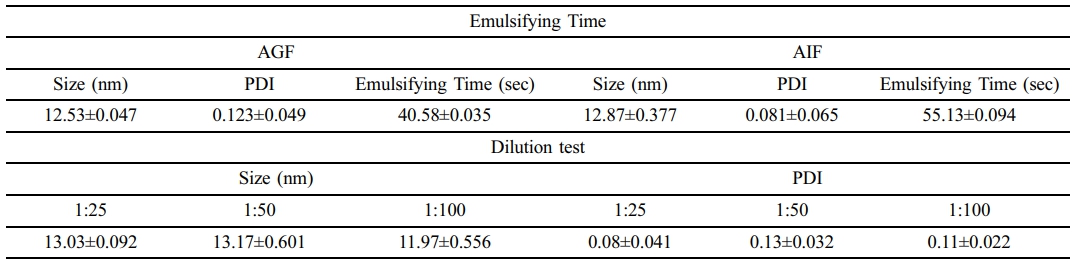
To evaluate the emulsification capability of the parsley-loaded SNEDDS in different solvent conditions and at varying dilution ratios, robustness of dilution and emulsification tests were performed. For the emulsification test, the parsley SNEDDS was dissolved in artificial gastric fluid (AGF) and artificial intestinal fluid (AIF). The formulations were stirred at 100 rpm, and the time required to form a well-dispersed nanoemulsion was recorded. The droplet size and PDI were subsequently measured. As shown in Table 3, parsley-loaded SNEDDS exhibited stable droplet size and PDI values in both AGF and AIF, with sizes of 12.53 nm and 12.87 nm, respectively, and emulsification times under one minute for each solvent. For the dilution stability test, parsley SNEDDS was diluted in deionized water at three different ratios (1:25, 1:50, and 1:100). Results indicated that the SNEDDS maintained stability across all dilution factors, with consistently small droplet sizes and favorable PDI values, demonstrating good dispersibility across conditions.
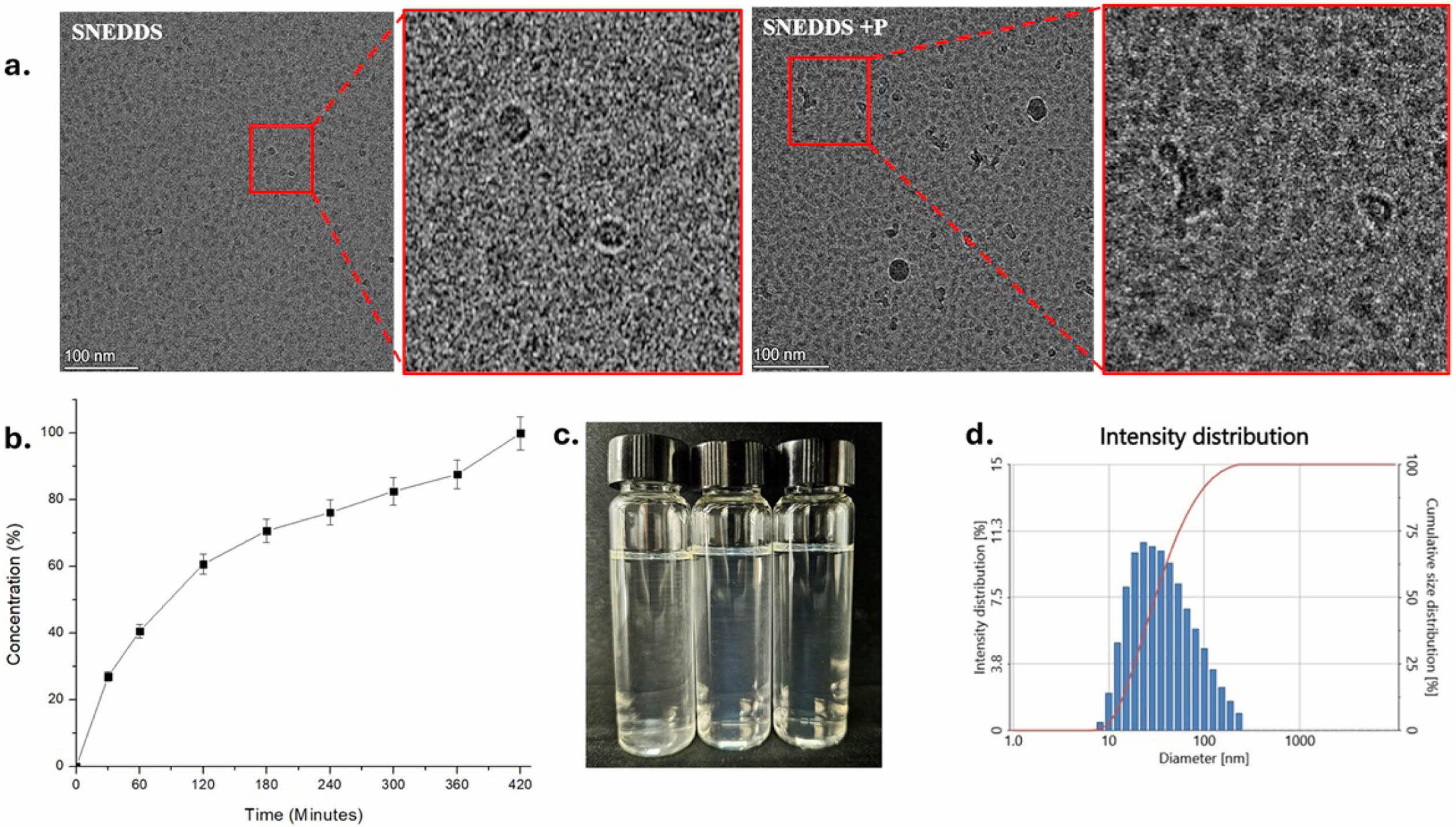
To further characterize the SNEDDS, cryo-TEM analysis was conducted using Cryo Field Emission TEM (Glacios, Thermo Fisher) operating at 200 kV. As shown in Figure 5(a), the droplet size observed in the TEM images was consistent with DLS results, confirming the size and PDI observations. The TEM data revealed small SNEDDS droplet sizes with excellent dispersibility. Based on these evaluations, the SNEDDS demonstrated an impressive nanoemulsion profile. Previous studies have shown that smaller droplet sizes are associated with enhanced drug performance and bioavailability.25 Smaller droplets also help overcome steric barriers in mucus penetration and contribute to increased stability by reducing interfacial tension.26
Regarding surface charge, the zeta potential of the SNEDDS was measured at an average of –21.22 mV, which is highly favorable. Zeta potentials between ±20 mV and ±30 mV are generally considered moderately stable and suitable for oral delivery systems.27 Additionally, the SNEDDS maintained consistent droplet sizes even after serial dilutions (25×, 50×, and 100×). The emulsification time required for the SNEDDS to disperse completely in AGF and AIF was under one minute, meeting the grade A standard for emulsification time.28
In vitro drug release studies were conducted to evaluate the ability of SNEDDS to facilitate drug permeation across a biological membrane. A 2 kDa MWCO dialysis membrane was employed to simulate the biological membrane of the gastrointestinal (GI) tract. Drug concentration in phosphate-buffered saline (PBS, pH 7) was monitored at predetermined time points (30, 60, 120, 180, 240, 300, 360, and 420 minutes). As illustrated in Figure 5(b), the release profile showed a rapid release phase within the first 60 minutes, likely due to the immediate release of surface-adsorbed drug molecules. Between 60 and 360 minutes, the release transitioned into a sustained phase as drug diffusion from within the droplets became the rate-limiting step. By 360 – 420 minutes, the release plateaued, approaching 100% drug release, indicating complete depletion of the drug reservoir in the SNEDDS formulation.
The biphasic release profile observed—an initial burst followed by a slower, sustained release—is advantageous for applications requiring immediate therapeutic levels with prolonged drug activity. This dual-phase release pattern, combined with the SNEDDS's stability, rapid emulsification, and consistent droplet size, underscores its potential as an efficient drug delivery system with enhanced bioavailability. These findings highlight the SNEDDS as a robust and promising platform for future drug delivery applications.
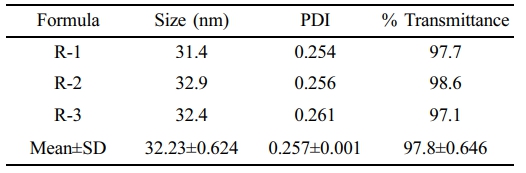
Stability Study. To evaluate the stability of the Parsley-SNEDDS formulation, we conducted a series of rigorous tests, including centrifugation, heating-cooling cycles, freezing-thawing cycles, and an endurance test. The centrifugation test assessed the kinetic stability of the SNEDDS, while the heating-cooling and freezing-thawing cycle tests evaluated the formulation's resilience to temperature variations. As illustrated in Figure 5(c), the Parsley-SNEDDS exhibited stability across all tests, with no phase separation observed after each procedure. Additionally, measurements of percent transmittance, particle size, and PDI following the stability tests confirmed the robustness of the formulation. Despite a slight increase in particle size, it remained under 50 nm, the PDI was maintained below 0.4, and the percent transmittance was 97.8%, as summarized in Table 4.
The results indicate that the SNEDDS formulation demonstrated excellent stability. Both the heating-cooling and freeze-thaw cycles, performed to assess thermodynamic stability, revealed no phase separation and preserved the original transparency of the SNEDDS. Similarly, the kinetic stability test using centrifugation yielded consistent results. Subsequent evaluations of particle size, PDI, and % transmittance showed only minor, statistically insignificant changes. Collectively, these findings confirm that the SNEDDS formulation exhibits robust thermodynamic and kinetic stability.
Herbal medicines are often administered as extracts or fractions, which frequently exhibit low bioavailability due to poor water solubility. SNEDDS provide a promising approach to enhance the bioavailability of herbal medicines. By forming nanoemulsions, SNEDDS formulations improve the solubility of herbal extracts, leading to increased absorption and bioavailability.29
In addition, SNEDDS are relatively simple to manufacture, offer convenient dosage forms that improve patient compliance, and demonstrate long-term stability.30
In this study, we developed and optimized SNEDDS for the n-hexane fraction of parsley (Petroselinum crispum), a herb renowned for its diverse therapeutic properties. Parsley is associated with numerous health benefits, including antioxidant activity, cardiovascular support, blood sugar regulation, urinary and kidney health promotion, anti-inflammatory effects, and digestive health enhancement.31-32
The fraction was incorporated into coconut oil, which served as the oil phase. Coconut oil not only served as a vehicle for the active fraction but also provided inherent therapeutic benefits, including antioxidant and antimicrobial properties. The formulation process was guided by a D-optimal design approach using Design Expert software (Version 8.0.6), which allowed for the systematic optimization of critical parameters.
The optimized parsley-SNEDDS demonstrated a highly favorable profile. The droplet size was 12.15 nm, well below the critical threshold of 20 nm, which facilitates improved penetration across epithelial and mucosal layers, including the gastrointestinal tract.33 The formulation's PDI was 0.178, indicating excellent uniformity and dispersion, as PDI values below 0.3 are considered optimal for nanoemulsions.34 The zeta potential averaged -21.22 mV, within the range that ensures stability and suggests enhanced interaction with the gastrointestinal tract for improved absorption.29 Cryo-TEM imaging further validated the uniformity and nanoscale size of the droplets, confirming the robustness of the formulation. In vitro drug release studies revealed a biphasic release pattern, with an initial rapid release phase followed by a sustained release phase, ultimately achieving 100% drug release after 420 minutes. This was achieved using a 2 kDa MWCO dialysis membrane designed to mimic physiological conditions.
Furthermore, stability testing under thermodynamic and kinetic conditions demonstrated that the parsley-SNEDDS maintained its size, dispersibility, and transparency, underscoring its long-term reliability. The ratio of surfactant to co-surfactant emerged as a critical determinant of nanoemulsion properties. Tween 80, used as the surfactant, displayed excellent compatibility with coconut oil, effectively emulsifying it in the aqueous phase.22
While Tween 80 is a non-ionic surfactant, the negative surface charge of the nanoemulsion can be attributed to hydroxyl ion adsorption from the aqueous phase or free fatty acids in the oil phase.35
The co-surfactant, PEG 400, played a synergistic role in enhancing the stability and performance of the formulation. PEG 400 provides steric stabilization, complementing the electrostatic stabilization offered by Tween 80. This dual mechanism helps prevent droplet coalescence by creating an interfacial barrier, reducing interfacial tension, and minimizing aggregation. The combination of Tween 80 and PEG 400 also contributed to the generation of smaller droplet sizes, further improving the nanoemulsion’s bioavailability.24 These findings highlight the potential of the parsley-SNEDDS formulation as a highly stable and effective delivery system for herbal medicine. The integration of a well-optimized surfactant-co-surfactant system and the therapeutic contributions of coconut oil underscore the formulation's suitability for enhancing the bioavailability of herbal extracts, offering a robust platform for future drug delivery applications.
|
Figure 1 2D and 3D Ternary contour plots illustrating the effects of oil, surfactant, and co-surfactant ratios on particle size, zeta potential, and polydispersity index (PDI). |
|
Figure 2 Ternary contour plots of the desirability profiles for varying ratios of oil, surfactant, and co-surfactant. |
|
Figure 3 Schematic protocol for the preparation of parsley SNEDDS formulation. |
|
Figure 4 Visual appearance of SNEDDS formulations: (a) before dilution; (b) after 1:25 dilution in deionized water, showing well-dispersed nanoemulsions. Diagram of the size intensity distribution profiles for (c) SNEDDS; (d) parsley SNEDDS. |
|
Figure 5 (a) Cryo-TEM images of SNEDDS and parsley-loaded SNEDDS, illustrating droplet morphology and size uniformity; (b) in vitro drug release profile of Parsley-SNEDDS; (c) visual appearance; (d) size intensity distribution of Parsley-SNEDDS after stability testing |
|
Table 1 Formulation Design and Key Parameter Results (particle size, zeta potential, and PDI) Generated Using Design-Expert® Software Version 8.0.6 |
|
Table 2 Droplet Size, Zeta Potential, Polydispersity Index (PDI), and Percent Transmittance Data for SNEDDS and Parsley-loaded SNEDDS |
|
Table 4 Droplet size, PDI, and Percent Transmittance Data for Parsley-SNEDDS After Stability Test |
In conclusion, this study successfully developed a SNEDDS capable of encapsulating the n-hexane fraction of parsley, achieving an excellent nanoemulsion profile. The formulation demonstrated small droplet size, high dispersibility, optimal surface charge, and robust stability under both kinetic and thermodynamic conditions. Based on these results, the SNEDDS formulation exhibits excellent potential for effective dispersion in the gastrointestinal tract, enhanced absorption, and ultimately improved bioavailability of the n-hexane fraction of parsley.
- 1. Mosihuzzaman, M. Herbal Medicine in Healthcare-an Overview. Nat. Prod. Commun. 2012, 7, DOI:10.1177/1934578X1200700628.
- 2. Firenzuoli, F.; Gori, L. Herbal Medicine Today: Clinical and Research Issues. Evidence‐Based Complement. Altern. Medicine 2007, 4, 37-40.
-

- 3. Khan, M. S. A.; Ahmad, I. Herbal Medicine: Current Trends and Future Prospects. In New look to Phytomedicine, Elsevier: London, 2019; pp 3-13.
- 4. Punoševac, M.; Radović, J.; Leković, A.; Kundaković-Vasović, T. A Review of Botanical Characteristics, Chemical Composition, Pharmacological Activity and Use of Parsley. Archiv. Pharm. 2021, 71, 177-196.
-

- 5. Suryani, S.; Zubaydah, W. O. S.; Sahumena, M. H.; Adawia, S.; Wahyuni, R.; Adjeng, A. N. T.; Nisa, M.; Kasmawati, H.; Ihsan, S.; Ruslin, R. Preparation and Characterization of Self-nanoemulsifying Drug Delivery System (SNEDDS) from Moringa Oleifera L. and Cassia alata L. Leaves Extracts, AIP Conf. Proc. 2019, 2197, 070011.
-

- 6. Saraf, S. Applications of Novel Drug Delivery System for Herbal Formulations. Fitoterapia 2010, 81, 680-689.
-

- 7. Pouton, C. W. Formulation of Self-emulsifying Drug Delivery Systems. Adv. Drug Delivery Rev. 1997, 25, 47-58.
-

- 8. Narang, A. S.; Delmarre, D.; Gao, D. Stable Drug Encapsulation in Micelles and Microemulsions. Int. J. Pharm. 2007, 345, 9-25.
-

- 9. Suhery, W. N.; Djohari, M.; Nur, N. R. Pengaruh Jenis Minyak Terhadap Karakterisasi Sifat Fisik dan Disolusi Self-Nanoemulsifying Drug Delivery System (SNEDDS) Asam Fenofibrat. JOPS 2023, 7, 1-9.
-

- 10. Hosny, K. M.; Alhakamy, N. A.; Sindi, A. M.; Khallaf, R. A., Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and in vivo Evaluation. Pharmaceutics 2020, 12, 1061.
-

- 11. Narayanankutty, A.; Illam, S. P.; Raghavamenon, A. C. Health Impacts of Different Edible Oils Prepared from Coconut (Cocos nucifera): A Comprehensive Review. Trends in Food Sci. Technol. 2018, 80, 1-7.
-

- 12. Esclapez, M.; García-Pérez, J. V.; Mulet, A.; Cárcel, J. Ultrasound-assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108-120.
-

- 13. Shafazila, T.; Lee, P. M.; Hung, L. K. Radical Scavenging Activities of Extract and Solvent-solvent Partition Fractions from Dendrobium Sonia “Red Bom” Flower, 2010 International Conference on Science and Social Research (CSSR 2010), IEEE, 2010, pp 762-765.
-

- 14. Hosny, K. M.; Rizg, W. Y.; Khallaf, R. A. Preparation and Optimization of in situ Gel Loaded with Rosuvastatin-ellagic Acid Nanotransfersomes to Enhance the Anti-proliferative Activity. Pharmaceutics 2020, 12, 263.
-

- 15. Nasr, A.; Gardouh, A.; Ghonaim, H.; Abdelghany, E.; Ghorab, M. Effect of Oils, Surfactants and Cosurfactants on Phase Behavior and Physicochemical Properties of Self-nanoemulsifying Drug Delivery System (SNEDDS) for Irbesartan and Olmesartan. Int J. Appl. Pharm. 2016, 8, 13-24.
-

- 16. Kanwal, T.; Saifullah, S.; ur Rehman, J.; Kawish, M.; Razzak, A.; Maharjan, R.; Imran, M.; Ali, I.; Roome, T.; Simjee, S. U. Design of Absorption Enhancer Containing Self-nanoemulsifying Drug Delivery System (SNEDDS) for Curcumin Improved Anti-cancer Activity and Oral Bioavailability. J. Mol. Liq. 2021, 324, 114774.
-

- 17. Balakumar, K.; Raghavan, C. V.; Abdu, S. Self Nanoemulsifying Drug Delivery System (SNEDDS) of Rosuvastatin Calcium: Design, Formulation, Bioavailability and Pharmacokinetic Evaluation. Colloids Surf., B 2013, 112, 337-343.
-

- 18. Elnaggar, Y. S.; El-Massik, M. A.; Abdallah, O. Y. Self-nanoemulsifying Drug Delivery Systems of Tamoxifen Citrate: Design and Optimization. Int. J. Pharm. 2009, 380, 133-141.
-

- 19. Wahyuningsih, I.; Putranti, W. Optimasi Perbandingan Tween 80 Dan Polietilenglikol 400 Pada Formula Self Nanoemulsifying Drug Delivery System (SNEDDS) Minyak Biji Jinten Hitam. Pharm. J. Indones. 2015, 12, 223-241.
-

- 20. Shukla, J. B.; Patel, S. J. Formulation and Evaluation of Self Micro Emulsifying System of Candesartan Cilexetil. Int. J. Pharm. Pharm. Sci. 2010, 2, 143-146.
- 21. Gupta, S.; Chavhan, S.; Sawant, K. K. Self-nanoemulsifying Drug Delivery System for Adefovir Dipivoxil: Design, Characterization, in vitro and ex vivo Evaluation. Colloids Surf. A 2011, 392, 145-155.
-

- 22. Sarheed, O.; Dibi, M.; Ramesh, K. V. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223.
-

- 23. Piriyaprasarth, S.; Sriamornsak, P.; Chansiri, G.; Promboot, W.; Imerb, U.; Sumpoung, D. Effect of Coconut Oil and Surfactants on Stability of Nanoemulsions. Adv. Mater. Res. 2012, 506, 429-432.
-

- 24. Miksusanti, E. F. A.; Bihurinin, A. H. B. Optimization of Tween 80 and PEG-400 Concentration in Indonesian Virgin Coconut Oil Nanoemulsion as Antibacterial Against Staphylococcus Aureus. Sains Malaysiana 2023, 52, 1259-1272.
-

- 25. Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y. H. Multifunctional Oral Delivery Systems for Enhanced Bioavailability of Therapeutic Peptides/proteins. Acta Pharmaceutica Sinica B 2019, 9, 902-922.
-

- 26. Buya, A. B.; Beloqui, A.; Memvanga, P. B.; Préat, V. Self-nano-emulsifying Drug-delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194.
-

- 27. Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F. K.; Badran, M. M.; Khan, A. A.; Alanazi, A. M.; Hussain, M. D. Evaluation of Self-nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-soluble Talinolol: Preparation, in vitro and in vivo Assessment. Front. Pharmacol. 2019, 10, 459.
-

- 28. Mohite, P.; Sule, S.; Pawar, A.; Alharbi, H. M.; Maitra, S.; Subramaniyan, V.; Kumarasamy, V.; Uti, D. E.; Ogbu, C. O.; Oodo, S. I. Development and Characterization of a Self-nano Emulsifying Drug Delivery System (SNEDDS) for Ornidazole to Improve Solubility and Oral Bioavailability of BCS Class II Drugs. Sci. Rep. 2024, 14, 27724.
-

- 29. Morakul, B. Self-nanoemulsifying Drug Delivery Systems (SNEDDS): an Advancement Technology for Oral Drug Delivery. Pharm. Sci. Asia 2020, 47, 205-220.
-

- 30. Baloch, J.; Sohail, M. F.; Sarwar, H. S.; Kiani, M. H.; Khan, G. M.; Jahan, S.; Rafay, M.; Chaudhry, M. T.; Yasinzai, M.; Shahnaz, G. Self-nanoemulsifying Drug Delivery System (SNEDDS) for Improved Oral Bioavailability of Chlorpromazine: in vitro and in vivo Evaluation. Medicina 2019, 55, 210.
-

- 31. Ganea, M.; Vicaș, L. G.; Gligor, O.; Sarac, I.; Onisan, E.; Nagy, C.; Moisa, C.; Ghitea, T. C. Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis. Molecules 2024, 29, 608.
-

- 32. Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Kamaly, O. M. A.; Jawhari, F. Z.; Imtara, H.; Grafov, A.; Bousta, D. The Potential of Parsley Polyphenols and Their Antioxidant Capacity to Help in the Treatment of Depression and Anxiety: An in vivo Subacute Study. Molecules 2021, 26, 2009.
-

- 33. Harmon, T. M.; Huang, J. Nano-Emulsion Formulations for Injection & Oral Administration. Drug Development & Delivery 2014, 14, 34-38.
- 34. Jusril, N. A.; Abu Bakar, S. I.; Khalil, K. A.; Md Saad, W. M.; Wen, N. K.; Adenan, M. I. Development and Optimization of Nanoemulsion from Ethanolic Extract of Centella asiatica (NanoSECA) Using D‐Optimal Mixture Design to Improve Blood‐Brain Barrier Permeability. Evidence‐Based Complementary and Alternative Medicine 2022, 2022, 3483511.
-

- 35. Zhang, C.; Li, B. Fabrication of Nanoemulsion Delivery System with High Bioaccessibility of Carotenoids from Lycium Barbarum by Spontaneous Emulsification. Food Sci. Nutr. 2022, 10, 2582-2589.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(3): 375-385
Published online May 25, 2025
- 10.7317/pk.2025.49.3.375
- Received on Jan 14, 2025
- Revised on Jan 24, 2025
- Accepted on Jan 29, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusion
- References
Shared
 Correspondence to
Correspondence to
- Yong-kyu Lee
-
*Department of Chemical & Biological Engineering, Korea National University of Transportation, Chungju 27470, Korea
**4D Convergence Technology Institute, Korea National University of Transportation, Jeungpyeong 27909, Korea - E-mail: leeyk@ut.ac.kr
- ORCID:
0000-0001-8336-3592








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.