- [Review]
- Zn2+-releasingBioactive Hydrogels for Endogenous Tissue Regeneration
*Department of Bioengineering and Nano-Bioengineering, Incheon National University,
Incheon 22012, Korea
**Research Center for Bio Materials & Process Development, Incheon National University,
Incheon 22012, Korea- 내인성 조직 재생을 위한 아연 이온 방출 생체활성 하이드로젤
*인천대학교 생명∙나노바이오 공학과, **인천대학교 바이오소재 및 공정개발 연구센터
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Bioactive hydrogels are widely used in endogenous tissue regeneration. Among these, Zn2+-releasing bioactive hydrogels have attracted attention due to their antibacterial properties, anti-inflammatory responses, angiogenesis, and collagen synthesis. Although various Zn2+-releasing biomaterials have been developed, they still face limitations, such as burst ion release. Therefore, strategies for sustained Zn2+ release are necessary. In this review, we highlight design strategies for biomaterials to enhance endogenous tissue regeneration, with a specific focus on Zn2+-releasing hydrogels. Our work emphasizes the therapeutic role of Zn2+ in wound healing and recent advancements in Zn2+-releasing biomaterials. Additionally, we explore strategies for sustained Zn2+ release to maximize therapeutic efficacy. By integrating these insights, this review offers a distinctive perspective on the development of advanced biomaterials for tissue regeneration.
생체활성 하이드로젤은 내인성 조직재생 향상을 위해 널리 사용되어 왔다. 이 중에서도 아연 이온 방출 생체활성 하이드로젤은 항균작용, 항염증작용, 신혈관형성, 콜라겐 합성을 증진시키는 특성을 가지고 있어 많은 주목을 받고 있다. 다양한 아연 이온 방출 생체재료들이 개발되고 있지만, 초기 빠른 이온 방출이라는 제한점을 가지고 있다. 따라서, 서방형 아연 이온 방출을 위한 다양한 개발 전략이 필요한 상황이다. 본 총설 논문에서는 내인성 조직 재생을 촉진하기 위한 생체재료 설계 전략을 다루며, 특히 아연 이온 방출 하이드로젤에 초점을 맞춘다. 아연 이온의 상처 치유 효과와 이를 활용한 생체재료의 최신 동향을 논의하며, 치료 효과를 극대화하기 위한 지속적인 이온 방출 전략을 제시한다. 이러한 통찰을 바탕으로, 본 논문은 조직 재생을 위한 차세대 생체재료 개발에 새로운 시각을 제공한다.
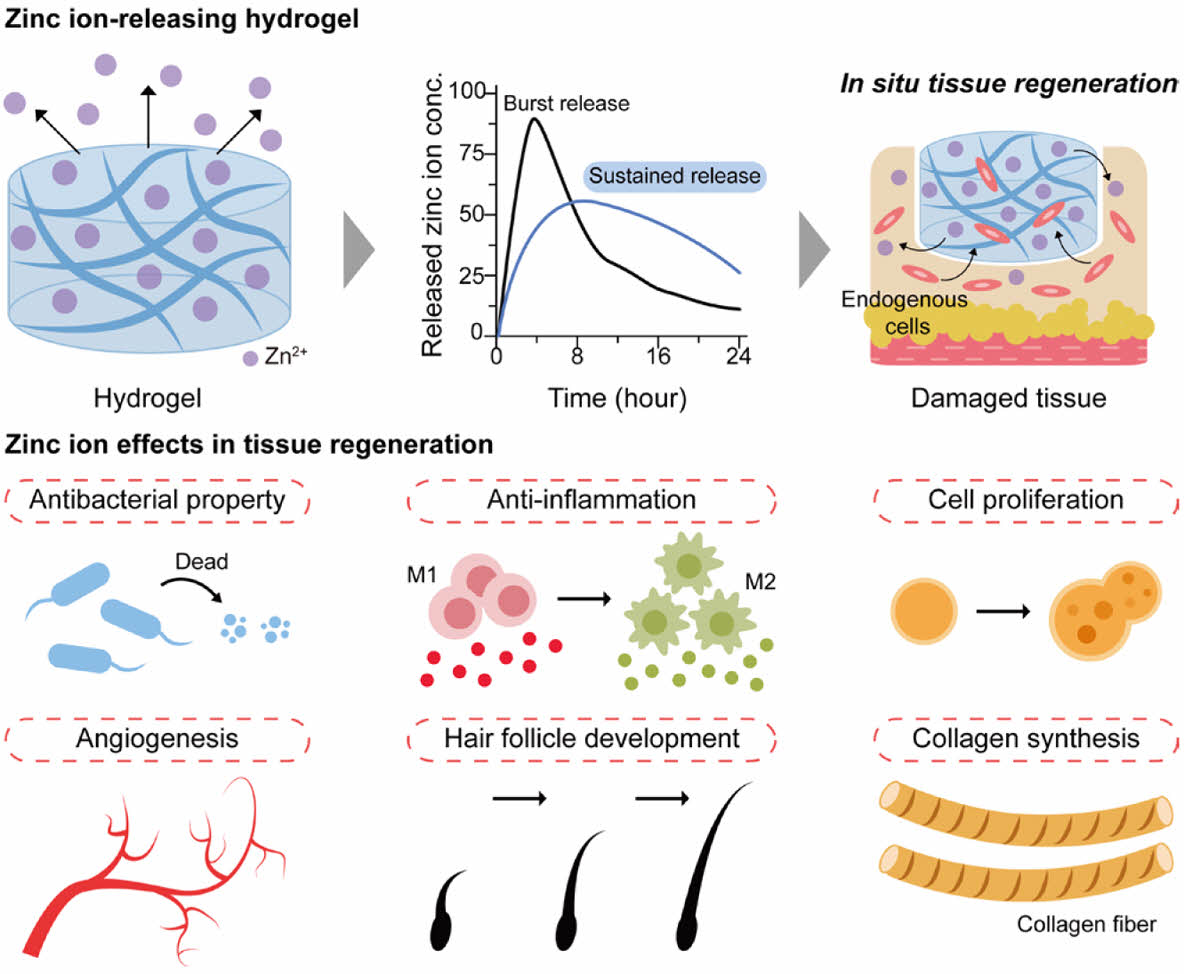
Zinc ions (Zn2+)-releasing hydrogels have been employed in the fields of endogenous tissue regeneration and wound healing, demonstrating a range of therapeutic effects. These effects encompass antibacterial properties, anti-inflammatory actions, stimulation of cell proliferation, promotion of angiogenesis, facilitation of hair follicle development, and enhancement of collagen synthesis.
Keywords: polymeric hydrogel, endogenous tissue regeneration, zinc ion, zinc peroxide.
This work was supported by the Incheon National University Research Grants in 2020 (2020-0418).
The authors declare that there is no conflict of interest.
Polymeric hydrogels are three-dimensional (3D) network structures consisting of hydrophilic polymers that can retain significant amounts of water.1,2
They provide an aqueous environment with high biocompatibility and show structural similarity to the natural extracellular matrix (ECM). Owing to these unique properties, hydrogels have been extensively studied in various biomedical fields including drug delivery, wound management, and tissue regeneration.
Hydrogels can be fabricated from various polymers via different crosslinking mechanisms. Polymers are generally categorized into natural and synthetic polymers, whereas crosslinking mechanisms fall into two broad categories: physical and chemical reactions.
Natural polymers are derived from the biological processes of natural materials, including polysaccharides and proteins.1
Examples include alginate, collagen, gelatin, chitosan, and hyaluronic acid (HA), as well as agarose and others.3
These polymers offer several advantages, such as cost-effectiveness, biodegradability, and bioactive properties. However, they also come with limitations, including challenges in sterilization, low mechanical strength, and significant variability between batches.4,5
In contrast, synthetic polymers are man-made materials designed for specific applications. Common examples include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), and polystyrene (PS).4,6,7
These highly customizable polymers have adjustable mechanical and degradation properties but exhibit limited interactions with cells and the ECM.
In addition to the type of polymer, the crosslinking mechanism significantly influences the properties and applications of hydrogels. Physically crosslinked hydrogels, frequently referred to as physical gels or reversible hydrogels, are created through non-covalent interactions, including ionic bonds, pH-responsive interactions, electrostatic forces, polymer entanglements, and various other methods.1,8
These hydrogels are recognized for their excellent biocompatibility and their ability to respond to external stimuli, such as temperature or ion concentration. However, they often demonstrate poor mechanical stability, especially under physiological conditions.9
Covalently crosslinked hydrogels, also known as chemical gels, are created through irreversible chemical reactions such as click chemistry, photo-crosslinking, Michael-type addition, and disulfide crosslinking. These hydrogels typically display superior mechanical stability, although they may raise biocompatibility concerns in certain applications.10,11
Polymeric hydrogels can be fabricated using various polymers and crosslinking methods. Depending on the specific application requirements, the appropriate choice of natural or synthetic polymers and the type of crosslinking methods must be carefully selected to enhance their properties and performance.
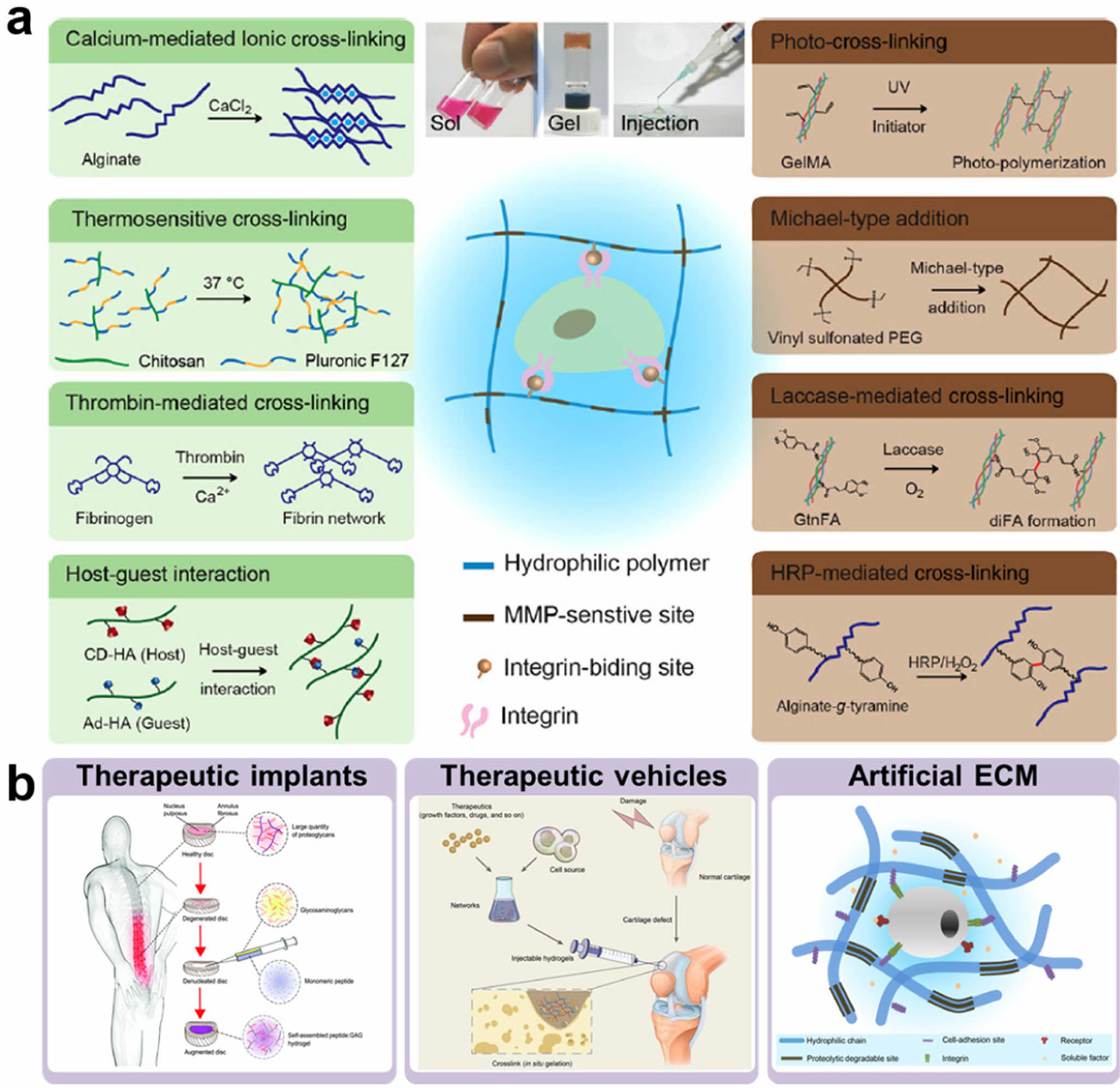
Among the various types of polymeric hydrogels, in situ cross-linkable hydrogels have attracted considerable attention as injectable matrices due to their sol-to-gel phase transition via various crosslinking strategies. These hydrogels can be produced using a variety of physical or chemical crosslinking reactions (Figure 1(a)).10,12
Unlike preformed hydrogels, in situ cross-linkable hydrogel has several advantages. They are minimally invasive to target sites, easy to handle, and capable of encapsulating bioactive molecules, such as cells, growth factors, and drugs.13,14
These unique properties make in situ hydrogels a promising tool for various biomedical applications, including therapeutic implants, drug delivery vehicles, and artificial ECM scaffolds (Figure 1(b)).10,15,16
|
Figure 1 Hydrogels for biomedical applications: (a) current strategies for hydrogel formation; (b) applications of in situ cross-linkable hydrogels in therapeutic implants, vehicles, and artificial ECM. Reproduced with permission from Refs. 10, 15-17, Park, K. M. et al., Encycl. Polym. Sci. Technol., 2017, 1-16. ©2017, John Wiley & Sons, Inc. Park, S. et al., Polymers, 2016, 8, 23. ©2016, MDPI. Miles, D. et al., J. Mat. Chem. B, 2016, 4, 3225-3231. ©2016, Royal Society of Chemistry. Wu, J. et al., Theranostics, 2020, 10, 9843-9864. ©2020, Ivyspring International Publisher. |
Various approaches have been explored for enhancing tissue regeneration. The traditional ex vivo tissue engineering method uses patient-derived cells along with biomaterial scaffolds and biological factors before being implanted into the patient’s body.18
However, this method has several limitations: complex cell culture processes, the need for compatible cell sources, and high costs.18
Endogenous tissue regeneration has emerged as a promising alternative for addressing these challenges. This approach modifies the microenvironment at the injury site by implanting biomaterials that facilitate host cell recruitment, self-organization, and differentiation. Compared to ex vivo methods, endogenous tissue regeneration offers a longer shelf life, fewer regulatory hurdles, and greater clinical applicability.19
The design strategies for bioactive materials for endogenous tissue regeneration are mainly categorized into biophysical and biochemical methods. Biophysical methods focus on the physical properties of biomaterials, including mechanical stiffness, porosity, degradation rate, and surface characteristics, which foster a microenvironment that is conducive to tissue regeneration (Figure 2(a)).2,19
For example, mechanical stiffness is crucial for angiogenesis. Matrices with moderate stiffness (800 Pa) enhanced cellular infiltration and angiogenic activity, and increased vascular endothelial growth factor receptor 2 (VEGFR2) compared to gels with either lower elasticity (700 Pa) or greater rigidity (900 kPa). In addition, porosity significantly affects blood vessel formation. Microporous scaffolds promote vascularization and tissue integration by providing an interconnected porous structure that facilitates cellular migration and tissue ingrowth.20
Biochemical methods integrate bioactive elements, such as cell adhesion ligands, drugs, genes, growth factors, and ions, to stimulate cellular activation and modulate cell behavior (Figure 2(b)). For instance, the incorporation of cell adhesion ligands can influence cell differentiation. The interaction between integrins (α3/α5β1) and biomaterials drives human mesenchymal stem cells (MSCs) to differentiate into the osteogenic lineage, demonstrating the capacity of biomaterials to regulate cellular phenotypes.21
Additionally, the incorporation of bioactive molecules plays a key role in immune modulation.22
The controlled release of immunomodulatory biomolecules from biomaterials can modify the local immune microenvironment, thereby directly enhancing tissue regeneration at the application site.
In conclusion, endogenous tissue regeneration offers a promising approach to overcoming the limitations of traditional ex vivo methods. By utilizing the biophysical and biochemical properties of biomaterials, this strategy can effectively modulate the microenvironment at the injury site, promoting cellular recruitment, immune modulation, angiogenesis, and tissue remodeling (Figure 2(c)).

|
Figure 2 Design approaches for in situ tissue regeneration: (a) biophysical properties (e.g., mechanical stiffness, porosity, degradation, surface charge, and wettability); (b) biochemical properties (e.g., cell adhesion ligands, cytokines, growth factors, and inorganic ions); (c) endogenous tissue regeneration through biophysical and biochemical modulation. |
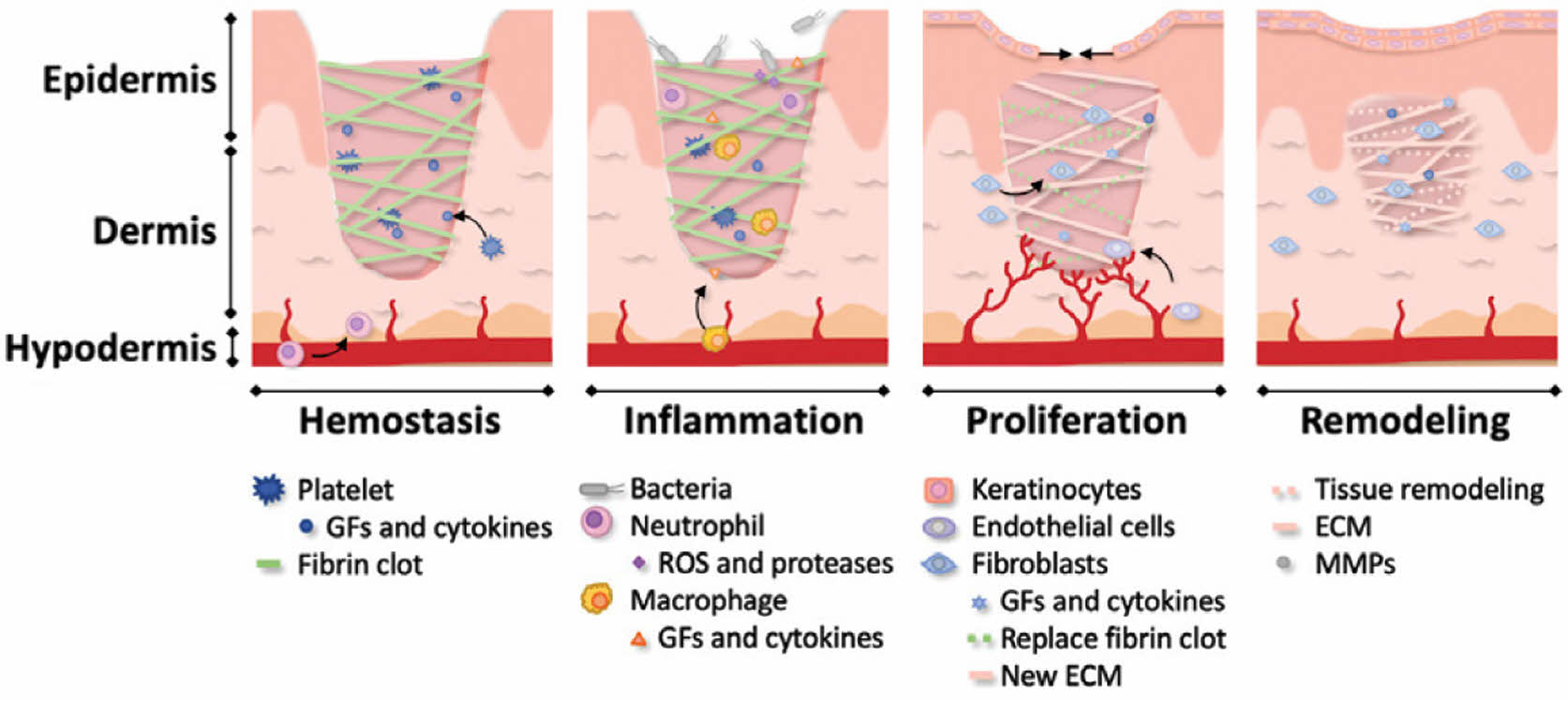
The skin is the largest organ in the body and performs various essential functions.23 It protects the body from external factors, regulates temperature, and detects sensations. However, wounds can easily occur owing to external stimuli, such as mechanical stimuli, thermal injuries, or radiation exposure. When these injuries occur, the body engages in a complex healing process that consists of four overlapping phases: 1) hemostasis, 2) inflammation, 3) proliferation, and 4) remodeling (Figure 3).24 The primary goal of wound healing is to restore tissue integrity effectively. Without proper treatment, these injuries may progress into chronic conditions, posing substantial obstacles to recovery. Therefore, identifying effective treatment strategies to promote tissue regeneration is critically important.2,24,25
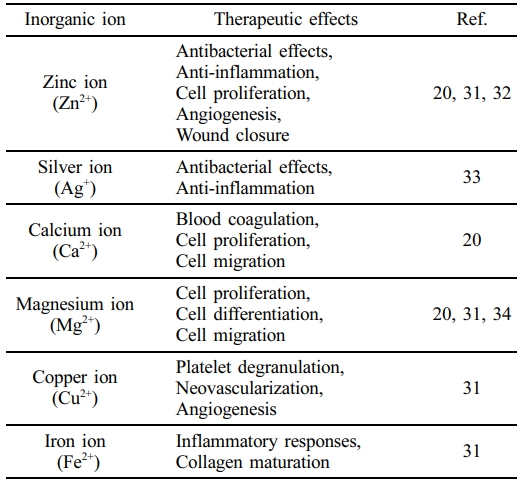
Various inorganic ions have recently garnered significant attention in wound treatment because of their critical role in the healing process (Table 1). Silver ion (Ag+) is well known for its antimicrobial properties and its ability to create an anti-inflammatory environment.27,28 Calcium ion (Ca2+) stimulates cell migration and proliferation, both of which are essential for tissue repair.29 Additionally, copper ion (Cu2+) promotes angiogenesis and improves wound healing.30 Among the reported inorganic ions, Zn2+ has been shown to regulate multiple stages of the wound healing process. It enhances hemostasis, exhibits antibacterial activity, immune modulation, cell proliferation, angiogenesis, supports hair follicle development, and stimulates collagen synthesis.31,32 Due to these therapeutic effects, Zn2+ has been widely used as a therapeutic agent for wound care and tissue regeneration.23
|
Figure 3 Schematic representation of the four phases of wound healing (hemostasis, inflammation, proliferation, and remodeling). Reproduced with permission from Ref. 26, Daikuara, L. et al., Adv. Funct. Mater., 2021, 32. ©2021, Wiley-VCH. |
It has been shown that Zn2+ plays a crucial role in the wound healing process (Figure 4).34
The specific mechanisms by which Zn2+ contributes to wound healing are as follows. 1) Zn2+ penetrates bacterial cell membranes, disrupting their structural integrity and ultimately leading to cell death.35-37 2) Zn2+ enhances the expression of anti-inflammatory cytokines and fosters an anti-inflammatory environment at injury sites.38 3) Zn2+ promotes the proliferation of various cell types by regulating hormones involved in cell division, facilitating the transition from the G1 phase to the S phase of the cell cycle.39 4) Zn2+ enhances endothelial cell viability and proliferation by increasing the expression of vascular endothelial growth factor A (VEGFA) and platelet-derived growth factor receptor alpha (PDGFRA). Additionally, Zn2+ promotes cytoskeletal reorganization and F-actin polymerization, facilitating cell migration.40-42 5) Zn2+ stimulates the migration of hair follicle stem cells near the wound.43,44
6) Zn2+ enhances collagen production, essential for maintaining the mechanical strength and elasticity of skin.45 Therefore, the sustained release of Zn2+ can significantly improve wound healing by continuous supply of Zn2+ at the injury site.46

|
Figure 4 The therapeutic effects of Zn2+ on the wound healing process. Reproduced with permission from Refs. 31, 47, Lin, P. et al., Nutrients, 2017, 10, 16. ©2017, MDPI. Srivastave, G. et al., Polymers, 2024, 16, 1280. ©2024, MDPI. |
Zn2+-releasing biomaterials have been utilized in various fields including tissue regeneration and medical treatment. These materials include zinc-based degradable metallic biomaterials, zinc oxide nanoparticles (ZnO NPs), zinc-containing metal-organic frameworks (MOFs), and zinc-mediated cross-linkable hydrogels (Figure 5(a)).48
Biodegradable Zn alloys have shown promise in bone fixation and vascular stent applications due to their slow degradation rates, mechanical stability, and biocompatibility. However, pure Zn metal exhibits a low mechanical stability and a very slow degradation rate. To overcome these challenges, researchers have developed zinc alloys that offer enhanced mechanical strength and favorable degradation characteristics. In comparison to Mg and Fe alloys, zinc-based alloys degrade more slowly than Mg alloys but at a faster rate than Fe alloys (Figure 5(b)). For instance, Xiwei Liu et al. developed Zn–Mg–Mn alloys that demonstrate improved mechanical properties, excellent hemocompatibility, and superior mechanical stability, making them suitable for orthopedic regeneration and cardiovascular stent applications.49
ZnO NPs are extensively used in tissue regeneration due to their antibacterial and wound healing properties (Figure 5(c)).50 These nanoparticles generate reactive oxygen species (ROS) and Zn2+, which penetrate bacterial membranes, disrupt their structure and interfere with DNA replication.51 Furthermore, the accumulation of excess Zn2+ within cells leads to cytotoxicity, causing membrane damage and apoptosis in cancer cells.52,53 ZnO NPs are favored for their affordability, compatibility with biological systems, and large surface area, making them essential in many biomedical applications.50,54 However, excessive production of ROS by ZnO NPs can lead to DNA damage and mitochondrial dysfunction.55
MOFs are widely utilized in drug delivery systems, cancer therapy, and bioimaging because of their high surface areas and pore volumes (Figure 5(d)).48,56,57 MOFs can be used in tissue regeneration to deliver anti-inflammatory drugs. For instance, zeolitic imidazolate MOF-8 (ZIF-8), a widely studied MOF, has been incorporated into nanofibrous scaffolds to improve diabetic wound healing via sustained Zn2+ release.58
Zn2+-mediated cross-linkable hydrogels can be formed using zinc-containing ceramics, zinc chloride, and zinc sulfate.59 These hydrogels enable the controlled release of Zn2+ and have a wide range of potential applications in regenerative medicine (Figure 5(e)). For example, alginate hydrogels crosslinked with divalent ions such as Zn2+ exhibit remarkable biocompatibility and structural similarity to the natural ECM, making them highly versatile for tissue regeneration.60 Despite their promise, Zn2+-releasing biomaterials face numerous challenges, including complex fabrication processes, the need for additional additives and modification, and burst ion release that may limit their effectiveness in clinical applications.

|
Figure 5 Recent trends and examples of Zn2+-releasing materials: (a) classification into biodegradable metals, ceramics, nanomaterials/organics, and Zn-based hydrogels; (b) Zn alloys for bone fixation and vascular stents; (c) nanoparticle-loaded scaffolds for antibacterial effects and wound healing; (d) Zn-based organic frameworks for cancer therapy; (e) alginate hydrogels for tissue regeneration. Reproduced with permission from Refs. 52, 60-62, Shin, H. et al., ACS Biomater. Sci. Eng., 2020, 6, 3430-3439. ©2020, American Chemical Society. Li, Y. et al., ACS Appl. Mater. Interfaces, 2017, 9, 16054-16062. ©2017, American Chemical Society. Hehrlein, C. et al., PLoS One, 2019, 14. ©2019, Public Library of Science. Mao, C. et al., ACS Nano, 2017, 11, 9010-9021. ©2017, American Chemical Society. |
Zn2+-doped bioglass, Zn2+-loaded bioactive ceramics, and ZnO NPs are commonly used for Zn2+ release.51,63,64 However, these materials exhibit a burst release profile of Zn2+, which limits their long-term effectiveness. Therefore, zinc peroxide (ZnO2) has attracted attention as a source of sustained-release Zn2+. ZnO2 decomposes and releases Zn2+, with hydrogen peroxide (H2O2) acting as an intermediate.65,66 The decomposition of ZnO2 proceeds via the following reactions:

These reactions allow ZnO2 to release Zn2+ more sustainably than other Zn2+ delivery sources.
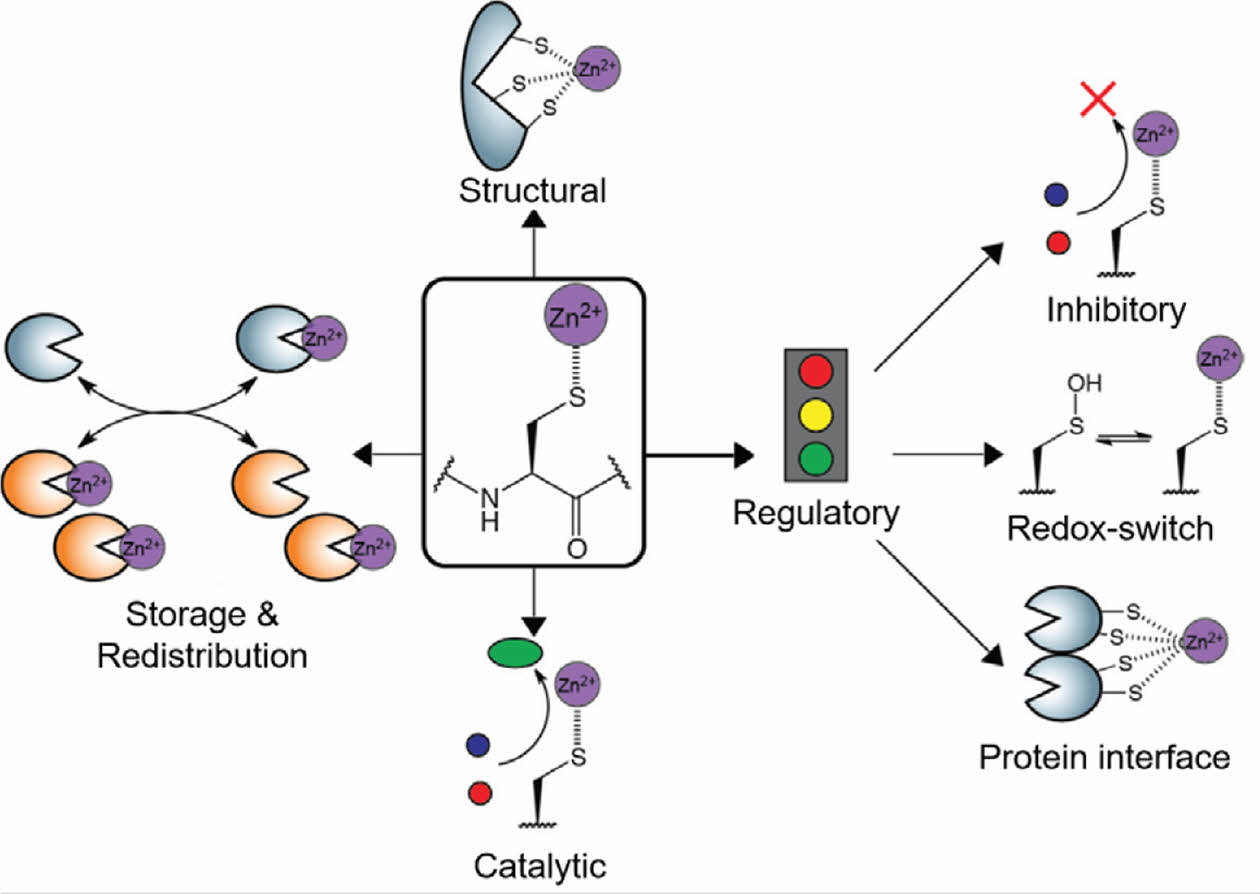
One of the most critical factors in the development of ion-releasing biomaterials is achieving a controlled and prolonged release of ions. Zn2+ has a high affinity for cysteine residues, which allows the formation of Zn2+-cysteine complexes (Figure 6). Specifically, these complexes play critical roles in maintaining the structural stability of biomolecules. Zn2+-cysteine complexes contribute to protein stabilization and DNA structural integrity. Zinc finger motifs, which are common domains in proteins and DNA, rely on these interactions to maintain structural integrity. Zn2+-cysteine interactions regulate protein function and activity.67,68 Additionally, cysteine-rich metallothioneins interact with Zn2+ to regulate intracellular Zn2+ concentration, thereby ensuring cellular homeostasis.69,70 The coordination between thiol groups and Zn2+ ensures a regulated and sustained Zn2+ release over time. This mechanism enhances the performance of Zn2+-releasing biomaterials and minimizes potential adverse effects. In conclusion, combining ZnO2 decomposition with the formation of a Zn2+-cysteine complex presents a promising strategy for achieving controlled and sustained Zn2+ levels in hydrogels, paving the way for advancements in biomaterials for long-term therapeutic applications.
|
Figure 6 Overview of the major functions of Zn2+-cysteine complexes. Reproduced with permission from Ref. 67, Pace, N. J. et al., Biomolecules, 2014, 4, 419-434. ©2014, MDPI. |
This review focuses on Zn2+-releasing bioactive hydrogels for endogenous tissue regeneration. Despite significant progress, many biomaterials still face challenges with rapid ion release, which limits their long-term efficacy. Consequently, recent research has shifted toward developing strategies for sustained and controlled ion delivery, including the ZnO2-mediated Zn2+ release system discussed in this article. Future studies should prioritize the development of biomaterials with prolonged ion release and multifunctional properties to enhance tissue regeneration. Additionally, a deeper understanding of the underlying biological mechanisms will be crucial for advancing biomaterial design. By integrating these strategies, researchers can pave the way for improved regenerative therapies.
- 1. Zhang, Y. S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627.
-

- 2. Kim, H. S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K. W. Advanced Drug Delivery Systems and Artificial Skin Grafts for Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 209-239.
-

- 3. Park, S.; Park, K. M. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers 2016, 8, 23.
-

- 4. Mariani, E.; Lisignoli, G.; Borzì, R. M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response?. Int. J. Mol. Sci. 2019, 20, 636.
-

- 5. Chen, Y. Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, 2019.
- 6. Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665.
-

- 7. Shrivastava, A. Introduction to Plastics Engineering; William Andrew: New York, 2018.
- 8. Mahinroosta, M.; Farsangi, Z. J.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42-55.
-

- 9. Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327-339.
-

- 10. Sivashanmugam, A.; Kumar, R. A.; Priya, M. V.; Nair, S. V.; Jayakumar, R. An Overview of Injectable Polymeric Hydrogels for Tissue Engineering. Eur. Polym. J. 2015, 72, 543-565.
-

- 11. Lee, J. H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, 27.
-

- 12. Dimatteo, R.; Darling, N. J.; Segura, T. In situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167-184.
-

- 13. Park, K. M.; Lewis, D.; Gerecht, S. Bioinspired Hydrogels to Engineer Cancer Microenvironments. Annu. Rev. Biomed. Eng. 2017, 19, 109-133.
-

- 14. Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situ Formation of Injectable Hydrogels for Chronic Wound Healing. J. Mat. Chem. B 2020, 8, 8768-8780.
-

- 15. Miles, D.; Mitchell, E.; Kapur, N.; Beales, P.; Wilcox, R. Peptide: Glycosaminoglycan Hybrid Hydrogels as An Injectable Intervention for Spinal Disc Degeneration. J. Mat. Chem. B 2016, 4, 3225-3231.
-

- 16. Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843.
-

- 17. Park, K. M.; Park, K. D. Injectable Hydrogels: Properties and Applications; John Wiley & Sons: New Jersey,2002.
- 18. Gaharwar, A. K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for In situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686-705.
-

- 19. Dias, J. R.; Ribeiro, N.; Baptista-Silva, S.; Costa-Pinto, A. R.; Alves, N.; Oliveira, A. L. In situ Enabling Approaches for Tissue Regeneration: Current Challenges and New Developments. Front. Bioeng. Biotechnol. 2020, 8, 85.
-

- 20. Masson‐Meyers, D. S.; Tayebi, L. Vascularization Strategies in Tissue Engineering Approaches for Soft Tissue Repair. J. Tissue Eng. Regen. Med. 2021, 15, 747-762.
-

- 21. Dhavalikar, P.; Robinson, A.; Lan, Z.; Jenkins, D.; Chwatko, M.; Salhadar, K.; Jose, A.; Kar, R.; Shoga, E.; Kannapiran, A. Review of Integrin‐targeting Biomaterials in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 2000795.
-

- 22. Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A. R. Immunomodulatory Bioactive Glasses for Tissue Regeneration. Acta Biomater. 2021, 133, 168-186.
-

- 23. Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of Adult Skin Wounds: Cellular Mechanisms and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2019, 146, 344-365.
-

- 24. Veith, A. P.; Henderson, K.; Spencer, A.; Sligar, A. D.; Baker, A. B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97-125.
-

- 25. Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B. B. Emerging and Innovative Approaches for Wound Healing and Skin Regeneration: Current Status and Advances. Biomaterials 2019, 216, 119267.
-

- 26. Daikuara, L. Y.; Chen, X.; Yue, Z.; Skropeta, D.; Wood, F. M.; Fear, M. W.; Wallace, G. G. 3D Bioprinting Constructs to Facilitate Skin Regeneration. Adv. Funct. Mater. 2022, 32, 2105080.
-

- 27. Naseri, S.; Lepry, W. C.; Nazhat, S. N. Bioactive Glasses in Wound Healing: Hope or Hype?. J. Mat. Chem. B 2017, 5, 6167-6174.
-

- 28. Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 3985-3999.
-

- 29. Wang, S.; Sun, Q.; Zhao, Y.; Huo, B. Distribution of Intracellular Calcium During Flow-induced Migration of RAW264. 7 Cells. MBM 2024, 2, 100012.
-

- 30. Liu, G.; Ye, S.; Li, Y.; Yang, J.; Wang, S.; Liu, Y.; Yang, S.; Tian, Y.; Yin, M.; Cheng, B. Copper Ions‐photo Dual‐crosslinked Alginate Hydrogel for Angiogenesis and Osteogenesis. J. Biomed. Mater. Res. Part A 2025, 113, e37790.
-

- 31. Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P. H.; Steinberg, S. M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16.
-

- 32. Yang, J.; Chu, Z.; Jiang, Y.; Zheng, W.; Sun, J.; Xu, L.; Ma, Y.; Wang, W.; Shao, M.; Qian, H. Multifunctional Hyaluronic Acid Microneedle Patch Embedded by Cerium/zinc‐based Composites for Accelerating Diabetes Wound Healing. Adv. Healthc. Mater. 2023, 12, 2300725.
-

- 33. Khansa, I.; Schoenbrunner, A. R.; Kraft, C. T.; Janis, J. E. Silver in Wound Care—friend or Foe?: A Comprehensive Review. PRS-Glob. Open 2019, 7, e2390.
-

- 34. Yang, F.; Xue, Y.; Wang, F.; Guo, D.; He, Y.; Zhao, X.; Yan, F.; Xu, Y.; Xia, D.; Liu, Y. Sustained Release of Magnesium and Zinc Ions Synergistically Accelerates Wound Healing. Bioact. Mater. 2023, 26, 88.
-

- 35. Abdulghani, S.; Mitchell, G. R. Biomaterials for in situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750.
-

- 36. Abebe, B.; Zereffa, E. A.; Tadesse, A.; Murthy, H. A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190.
-

- 37. Ijaz, M.; Zafar, M.; Islam, A.; Afsheen, S.; Iqbal, T. A Review on Antibacterial Properties of Biologically Synthesized Zinc Oxide Nanostructures. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2815-2826.
-

- 38. Liang, Y.; Wang, M.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Facile Synthesis of ZnO QDs@ GO-CS Hydrogel for Synergetic Antibacterial Applications and Enhanced Wound Healing. Chem. Eng. J. 2019, 378, 122043.
-

- 39. Holtzen, S. E.; Navid, E.; Kainov, J. D.; Palmer, A. E. Transient Zn2+ Deficiency Induces Replication Stress and Compromises Daughter Cell Proliferation. Proc. Natl. Acad. Sci. 2024, 121, e2321216121.
-

- 40. Zhu, D.; Su, Y.; Zheng, Y.; Fu, B.; Tang, L.; Qin, Y.-X. Zinc Regulates Vascular Endothelial Cell Activity Through Zinc-sensing Receptor ZnR/GPR39. Am. J. Physiol.-Cell Physiol. 2018, 314, C404-C414.
-

- 41. Li, Y.; Ma, T.; Zhu, X.; Zhang, M.; Zhao, L.; Wang, P.; Liang, J. Zinc Improves Neurological Recovery by Promoting Angiogenesis via the Astrocyte‐mediated HIF‐1α/VEGF Signaling Pathway in Experimental Stroke. CNS Neurosci. Ther. 2022, 28, 1790-1799.
-

- 42. Sreenivasamurthy, S. A.; Akhter, F. F.; Akhter, A.; Su, Y.; Zhu, D. Cellular Mechanisms of Biodegradable Zinc and Magnesium Materials on Promoting Angiogenesis. Biomater. Adv. 2022, 139, 213023.
-

- 43. Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn Doped Hollow Mesoporous Silica/polycaprolactone Electrospun Membranes with Enhanced Hair Follicle Regeneration and Antibacterial Activity for Wound Healing. Nanoscale 2019, 11, 6315-6333.
-

- 44. Zhang, Z.; Li, W.; Chang, D.; Wei, Z.; Wang, E.; Yu, J.; Xu, Y.; Que, Y.; Chen, Y.; Fan, C. A Combination Therapy for Androgenic Alopecia Based on Quercetin and Zinc/copper Dual-doped Mesoporous Silica Nanocomposite Microneedle Patch. Bioact. Mater. 2023, 24, 81-95.
-

- 45. Molenda, M.; Kolmas, J. The Role of Zinc in Bone Tissue Health and Regeneration—a Review. Biol. Trace Elem. Res. 2023, 201, 5640-5651.
-

- 46. Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid‐conjugated PDA Hydrogel Through Dual‐light Triggered ROS and Membrane Permeability. Small 2019, 15, 1900322.
-

- 47. Srivastava, G. K.; Martinez-Rodriguez, S.; Md Fadilah, N. I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M. B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280.
-

- 48. Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428-441.
-

- 49. Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn–Mg Alloy for Future Biodegradable Metals Application. Mater. Des. 2016, 94, 95-104.
-

- 50. Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562.
-

- 51. Wiesmann, N.; Mendler, S.; Buhr, C. R.; Ritz, U.; Kämmerer, P. W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials. Biomedicines2021, 9, 1462.
-

- 52. Shin, H.; Na, K. Cancer-targetable pH-sensitive Zinc-based Immunomodulators Combined with Photodynamic Therapy for in situ Vaccination. ACS Biomater. Sci. Eng. 2020, 6, 3430-3439.
-

- 53. Bendellaa, M.; Lelièvre, P.; Coll, J. L.; Sancey, L.; Deniaud, A.; Busser, B. Roles of Zinc in Cancers: From Altered Metabolism to Therapeutic Applications. Int. J. Cancer 2024, 154, 7-20.
-

- 54. Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 580.
-

- 55. Zhuo, L.-B.; Liu, Y.-M.; Jiang, Y.; Yan, Z. Zinc Oxide Nanoparticles Induce Acute Lung Injury via Oxidative Stress-mediated Mitochondrial Damage and NLRP3 Inflammasome Activation: In vitro and In vivo Studies. Environ. Pollut. 2024, 341, 122950.
-

- 56. Han, D.; Li, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K. W. K.; Li, C.; Cui, Z.; Liang, Y. Rapid Bacteria Trapping and Killing of Metal-organic Frameworks Strengthened Photo-responsive Hydrogel for Rapid Tissue Repair of Bacterial Infected Wounds. Chem. Eng. J. 2020, 396, 125194.
-

- 57. Song, M.-R.; Li, D.-Y.; Nian, F.-Y.; Xue, J.-P.; Chen, J.-J. Zeolitic Imidazolate Metal Organic Framework-8 as An Efficient pH-controlled Delivery Vehicle for Zinc Phthalocyanine in Photodynamic Therapy. J. Mater. Sci. 2018, 53, 2351-2361.
-

- 58. Wang, Y.; Ying, T.; Li, J.; Xu, Y.; Wang, R.; Ke, Q.; Shen, S. G.; Xu, H.; Lin, K. Hierarchical Micro/nanofibrous Scaffolds Incorporated with Curcumin and Zinc Ion Eutectic Metal Organic Frameworks for Enhanced Diabetic Wound Healing via Anti-oxidant and Anti-inflammatory Activities. Chem. Eng. J. 2020, 402, 126273.
-

- 59. Ju, Y.; Zeng, H.; Ye, X.; Dai, M.; Fang, B.; Liu, L. Zn2+ Incorporated Composite Polysaccharide Microspheres for Sustained Growth Factor Release and Wound Healing. Mater. Today Bio 2023, 22, 100739.
-

- 60. Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054-16062.
-

- 61. Hehrlein, C.; Schorch, B.; Kress, N.; Arab, A.; von Zur Mühlen, C.; Bode, C.; Epting, T.; Haberstroh, J.; Mey, L.; Schwarzbach, H. Zn-alloy Provides a Novel Platform for Mechanically Stable Bioresorbable Vascular Stents. PLoS One 2019, 14, e0209111.
-

- 62. Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K. W. K.; Pan, H.; Wang, X.; Chu, P. K.; Wu, S. Photo-inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010-9021.
-

- 63. Rajzer, I.; Dziadek, M.; Kurowska, A.; Cholewa-Kowalska, K.; Ziąbka, M.; Menaszek, E.; Douglas, T. E. Electrospun Polycaprolactone Membranes with Zn-doped Bioglass for Nasal Tissues Treatment. J. Mater. Sci.-Mater. Med. 2019, 30, 1-11.
-

- 64. Huang, X.; Huang, D.; Zhu, T.; Yu, X.; Xu, K.; Li, H.; Qu, H.; Zhou, Z.; Cheng, K.; Wen, W. Sustained Zinc Release in Cooperation with CaP Scaffold Promoted Bone Regeneration via Directing Stem Cell Fate and Triggering a Pro-healing Immune Stimuli. J. Nanobiotechnol. 2021, 19, 1-20.
-

- 65. Bergs, C.; Brück, L.; Rosencrantz, R. R.; Conrads, G.; Elling, L.; Pich, A. Biofunctionalized Zinc Peroxide (ZnO2) Nanoparticles as Active Oxygen Sources and Antibacterial Agents. RSC Adv. 2017, 7, 38998-39010.
-

- 66. Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A. A.; Yar, M.; ur Rehman, I. A Study on the Effect of Zinc Oxide and Zinc Peroxide Nanoparticles to Enhance Angiogenesis-pro-angiogenic Grafts for Tissue Regeneration Applications. Mater. Des. 2017, 132, 409-418.
-

- 67. Pace, N. J.; Weerapana, E. Zinc-binding Cysteines: Diverse Functions and Structural Motifs. Biomolecules 2014, 4, 419-434.
-

- 68. Burger, N.; Mittenbühler, M. J.; Xiao, H.; Shin, S.; Wei, S. M.; Henze, E. K.; Schindler, S.; Mehravar, S.; Wood, D. M.; Petrocelli, J. J. The Human Zinc-binding Cysteine Proteome. Cell 2024, 188, 832-850.
-

- 69. Kocyła, A.; Tran, J. B.; Krężel, A. Galvanization of Protein–protein Interactions in a Dynamic Zinc Interactome. Trends Biochem.Sci. 2021, 46, 64-79.
-

- 70. Sangeetha, V.; Dutta, S.; Moses, J.; Anandharamakrishnan, C. Zinc Nutrition and Human Health: Overview and Implications. eFood 2022, 3, e17.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(4): 387-394
Published online Jul 25, 2025
- 10.7317/pk.2025.49.4.387
- Received on Jan 30, 2025
- Revised on Feb 27, 2025
- Accepted on Feb 27, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Bioactive Hydrogels for Biomedical Applications
Design Strategies to Engineer Biomaterials for Endogenous Tissue Regeneration
Therapeutic Effects of Inorganic Ions for Skin Wound Healing
The Mechanisms of Zn2+in Wound Healing
Recent Trends in Zn2+-releasing Biomaterials
Zinc Peroxide-mediated Zn2+-releasing System
Conclusions and Future Direction
- References
Shared
 Correspondence to
Correspondence to
- Kyung Min Park
-
*Department of Bioengineering and Nano-Bioengineering, Incheon National University,
Incheon 22012, Korea
**Research Center for Bio Materials & Process Development, Incheon National University,
Incheon 22012, Korea - E-mail: kmpark@inu.ac.kr
- ORCID:
0000-0002-2216-2612









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.