- Flame Retardant (FR) and Antimicrobial Properties of Bifunctional Polyguanidine Salt Additives for Polypropylene (PP) Membrane Materials
Bowen Lv, Dongqing Liu†
 , Tian Xu, Yuxuan Cheng, Sun Zhou, Mengyuan Zhang, and Qinxing Xie
, Tian Xu, Yuxuan Cheng, Sun Zhou, Mengyuan Zhang, and Qinxing XieState Key Laboratory of Hollow Fiber Membrane Materials and Processes, Key Laboratory of Materials for Energy Storage, School of Material Science and Engineering, Tiangong University, Tianjin 300387, China
- Polypropylene(PP) 멤브레인 소재를 위한 이중 기능성 폴리구아니딘 염 첨가제의 난연 및 향균 특성 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Two polymeric additives, polyhexamethyl guanidine-diphenyl hypophosphite (DPPA-PHMG) and polyhexamethyl guanidine-chlorobridging acid (HA-PHMG), were prepared to overcome aqueous solubility of polyhexamethyl guanidine (PHMG) through salinization with two acidic flame retardant intermediates, diphenyl hypophosphite and chlorobridging acid. Melting points of two water-insoluble organic salts were 176.49 ℃ and 172.20 ℃ tested by TG, which were both higher than PHMG’s and brought better thermostability for the starting polymer. Their polypropylene (PP) blend films achieved 99.9% bactericidal rate for Escherichia coli and their suitable content improved flame-retardant performance in combustion experiments. PHMG serves as a useful platform for development of multifunctional additives.
Two polymer additives, poly(hexamethylguanidine)-diphenyl phosphite (DPPA-PHMG) and poly(hexamethylguanidine)-chlorobridging acid (HA-PHMG), were prepared in the study, and the melting points of the two water-insoluble organic salts were 176.49 ℃ and 172.20 ℃ test by TG, which were higher than the melting point of PHMG, and brought better thermostability for the starting polymer. The polypropylene (PP) blendedv films achieved a bactericidal tare of 99% against E coli.
Keywords: di-functional additives, bacteria inhibitor, flame retardant, polyhexamethyl guanidine salt.
The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51373119), Tianjin Key Projects of New Materials Science and Technology (17ZXCLGX00050).
As the emphasis on public health and safety requirements, the desire for bacteriostatic plastic and rubber became stronger in medical and public goods manufacturing owing to their inhibition to destruction of microorganisms and pathogens spreading.1-3 That reduces infection and makes more healthy life.4,5
Many long-lasting antibacterial polymer additives had been reported, including inorganic nano-particles, reactive organic anti-bactericides, metal-organic skeletons, and cationic polymers.6,7 Organic antimicrobial agents gradually became popular in industry field due to the advantages of facile process, enduring effect coming from good compatibility and environmental safety because of easy degradation in nature.8 Among them, oligomers of quaternary ammonium and phosphonium were confirmed quite effective, they could be introduced to polymer surfaces or matrices through blend, grafting, coupling, and other reactions, to disrupt proteins of cell wall of prokaryotic organisms when contact with them.9 Chen et al.10 developed a series of water-insoluble hyperbicycle-based biocidic complexes using poly-L-lysine (HBPL) and phosphate surfactant based on non-covalent superbranched interactions. The complexes showed excellent bactericidal ability to S. aureus even as small as 0.5 wt% in poly(ɛ-caprolactone) film. Qiu et al.11 coated cationized polyquaternary ammonium salt-containing skin through co-deposition of catechol and polyethyleneimine (PEI) on a surface of polypropylene (PP) microfiltration membranes, which obtained 95% inhibition to Gram-positive S. aureus.
Some ammonium cation-containing compounds not only possess antibacterial activity but also are effective flame retardants since some N-contained groups, such as guanidine salt and iminazole have the function of flame retardant.12 Zhao et al.13 separately impregnated boric acid and amidinourea phosphate into insulating paper and they respectively achieved 18.9% and 30.97% carbon residue, which are significantly higher than 1.97% of the original sample. When 10%-20% boric acid in their mixture, double salt between boric acid and amidinourea phosphate formed and could achieve the best flame retardant effect. Wan et al.14 grafted guanidinium tetramethylene phosphine acetate onto cotton fibers to fabricate a flame-retardant fiber, which gave a 45.5% LOI even after 50 washing cycles. Fang et al.15 constructed a multifunctional coat through layer-by-layer phytic acid and chitosan assembly on cotton fabric and then sprayed divalent Cu2+ and polydimethylsiloxane (PDMS). The coating layer was hydrophobic, flame retardant and antibacterial with a water contact angle of 150°, 32% LOI and 99% bacteriostatic rate for E. coil and S. aureus. It illustrated that plenty of ammonium cations on surface would provide the polymer both antibacterial and flame-retardant capacity.
PP, as a widely used polymer material, exhibits many beneficial properties, such as low density, high thermal stability, chemical resistance, good processability and recyclability,16 Tensile strength of commercial PP ranges from 27 to 40 MPa, which makes it a good building packaging material.17 The melting point of PP is about 160 ℃ to 180 ℃,18 depending on its crystallinity. However, side methyl groups bring low structural regularity and crystallinity.19 In addition, PP can be widely applied in medical area due to biocompatibility and non-toxicity.20 The fully aliphatic hydrocarbon skeleton makes it low decomposition speed and high flammability compared to cotton materials.21,22 However, the introduction of flame retardants, such as montmorillonite and C60, can greatly improve flame retardant performance through increasing limiting oxygen index (LOI) and ignition time.23,24
Polyhexamethylene guanidine hydrochloride (PHMG), is a kind of antimicrobial water-soluble oligomer with amino groups that can dissociate into ammonium cations.25,26 This provides a potential to be developed into multi-functional polymeric additives. Here, two water-insoluble organic salts were prepared by salinization reaction of PHMG and organic acids as the additives for PP. The structure of salts were characterized and they were blended with PP to prepare masterbatches through melting granulation. The bactericidal and flame retardant properties of the 2 salts were evaluated after masterbatches were prepared into PP films through thermally induced phase separation method. It was confirmed that suitable addition could provide PP good flame retardancy and 3 wt% addition could achieve 99.99% inhibition to E. coil and S. aureus. In plate count method test.
Materials and Instruments.Materials. PHMG was obtained from Hunan Xuelian Fine Chemical Co., Ltd. Chlorobridged acid (HA) and polypropylene (PP) were acquired from Tianjin Maidin Science and Technology Co., Ltd. Diphenylphosphinic acid (DPPA) was sourced from J&K Scientific. Beef paste, peptone, and agar powder were provided by Beijing Auboxing Biotechnology Co. Dioctyl phthalate (DOP) Source Tianjin Guangfu Science and Technology Development Co.
Instruments: Chemical structures of samples were analyzed by a Nicolet iS50 Fourier Transform Infrared Spectrometer (Thermo Fisher, USA) and a 1H NMR was measured by 400M03 liquid NMR (Bruker, USA) using d-DMSO as a solvent. Thermophysical properties were analyzed by TG 209 thermogravimetric analyzer and DSC204F differential scanning calorimeter (NETZSCH, Germany). Functional masterbatch and melt samples were extruded and pelletized by a small single-screw extruder (SJ-15, Jiangsu Orode Machinery Co., Ltd.). Combustion properties of samples were evaluated by an oxygen index meter (JF-3, Jiangning Analytical Instrument Factory, China), a vertical combustion meter (CFZ-1, Jiangning Analytical Instrument Co., Ltd., China), and a conical calorimeter (PX-07-007, Suzhou Phoenix Quality Inspection Co., Ltd.).
Synthesis of Polyhexamethylene Guanidine Salts. DPPA aqueous solution (7.55 g in 25 g water) was dropped in PHMG (53 g) saturated aqueous solution and stirred for 4 h. The white precipitate was vacuum-filtered and washed with deionized water. The filtration cake was dried at 40 ℃ to give 5.88 g white powder, DPPA-PHMG with a yield of 53.50%.
Similarly, HA-PHMG salt was synthesized under the same procedure of DPPA-PHMG by 2.93 g HA with a yield of 57.73%.
Preparation of Functional PP Masterbatch. DPPA-PHMG 1.0 g, PP masterbatch 98.5 g and 0.5 g antioxidant 1010 were mixed and then pelletized by a single-screw extruder with a screw speed of 50 r/min, heating temperature of 200 ℃ and cooling temperature of 30 ℃. The feeding and rotating speeds were 11.4 r/min. The as-produced masterbatch was dried at 40 ℃ for 4 h.
HA-PHMG/PP blend masterbatch was prepared under the same procedure.
Preparation of Functional PP Membrane. 2 g PP-DPPA-PHMG masterbatch and 8 g DOP were mixed and heated at 200 ℃ under N2 to complete melt and stood for 1 h. The melt was poured onto a smooth iron plate and scraped into a thin film by a casting stick of 200 μm edge thickness. The film was then put into 25 ℃ DI water and carefully scraped off after cooling down, which was washed by and immersed in anhydrous ethanol for further use.
PP-HA-PHMG membrane was prepared under the same procedure.
Anti-bacterial Assessment. The antimicrobial performance of PP composite film was evaluated using E. coli as a target by plate counting method in National standard of P.R. China (GB/T20944.1: 2007).27
Flame Retardant Performance Test. The PP masterbatch is heated to 225 ℃ under nitrogen, stirred for 2 h and then allowed to stand at 225 ℃ for 1 h. The melt is then poured into silicone oil-coated molds and cooled to room temperature. Flame retardancy of strips is evaluated by vertical flame test of National standard of P.R. China (GB UL-94).
Synthesis and Structural Characterization of Additives. The salinization between PHMG and acids produced organic salts, DPPA-PHMG and HA-PHMG, which were both water-insoluble white powders (Scheme 1). The optimal ratio of PHMG to acid was 1:1 for two acids with the best yield of 57.73% and 53.5% for DPPA-PHMG and HA-PHMG, respectively.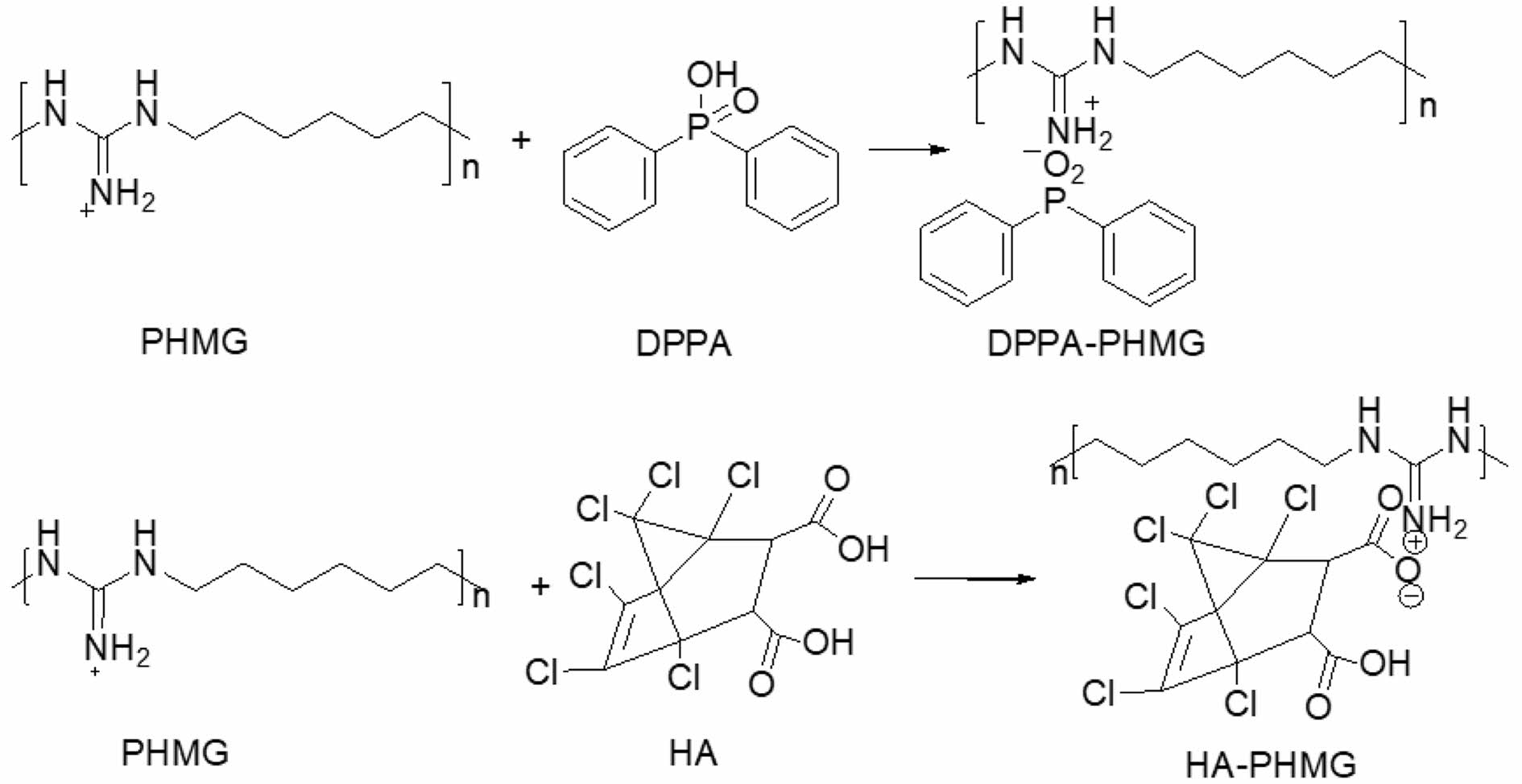
Scheme 1. Synthesis of DPPA-PHMG and HA-PHMG
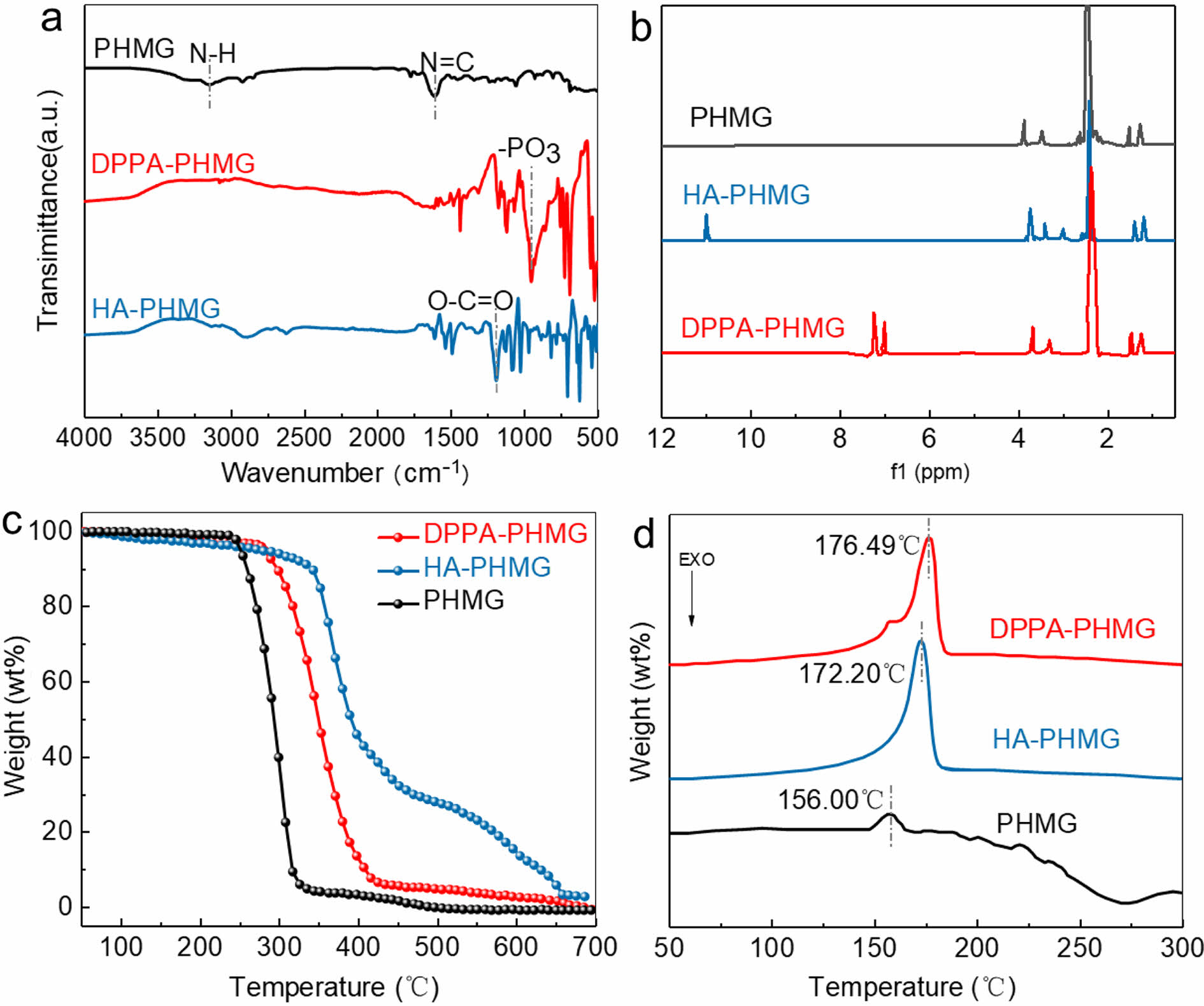
FTIR and 1H NMR characterized the chemical structures of DPPA-PHMG and HA-PHMG. In FTIR spectra (Figure 1(a)), the broad peak at 3259 cm-1 in PHMG curve was -N-H stretching vibration, which disappeared in curves of salts. The peak at 1620 cm-1 was stretching vibration of -N=C-, which was inconspicuous in both spectra of salts since -C=N-H in guanidine group took part in salinization and covered by acids sections. Absorption peaks at 1186 cm-1 in HA-PHMG and at 954 cm-1 in DPPA-PHMG spectrum were separately attributed to amide bond and hypophosphite groups, which illustrated the existence of HA and DPPA parts in new compounds. In 1H NMR spectra (Figure 1(b)), PHMG possessed a doublet at 1.43 ppm of 8 CH2 hexamethylene protons non-directly linking to N. The singlet at 3.09 ppm is 4 protons of CH2 linking to guanidine group. The peak at 3.24 ppm in HA-PHMG curve was CH2 protons in HA skeleton and the one at 11.4 ppm was carboxyl proton. In DPPA-PHMG curve, the singlet at 7.33 ppm is 4 N-linked CH2 protons. The twin peaks at 7.33 ppm and 7.69 ppm are benzene ring proton peaks. Both FTIR and 1H NMR confirmed the chemical structure of DPPA-PHMG and HA-PHMG. TG curves displayed that the onset and maximum decomposition temperatures of salts were both higher than those of PHMG (Figure 1(c)), which indicated their higher thermostability than PHMG. Although introduction of DPPA and HA destroyed intermolecular hydrogen bonds between PHMG chains, they increased molecular weight and enhanced new driving force of intermolecular interaction, involving p-p stacking and polar attraction, which led to higher thermostability. DSC curves (Figure 1(d)) showed that melting points of DPPA-PHMG and HA-PHMG were 176.49 ℃ and 172.20 ℃, which were higher than those of PHMG, 156.00 ℃. Since P, Cl and benzene cycle are all factors to increase decomposition temperature of materials, their introduction correspondingly increased melting points.
Preparation and Analyzation of Masterbatches. Table 1 listed recipes of DPPA-PHMG and HA-PHMG masterbatch and the best extrusion granulation parameters: screw speed 50 r/min, temperature of charging port 200 ℃, cooling temperature of feeding process 30 ℃ and rotational speed of pelletizing machine 11.4 r/min. When the additive content of HA was more than 5%, phase separation emerged and the master match was unsuitable for later usage. Therefore, 1% and 3% were used to prepare films to evaluate antibacterial and flame retardant.
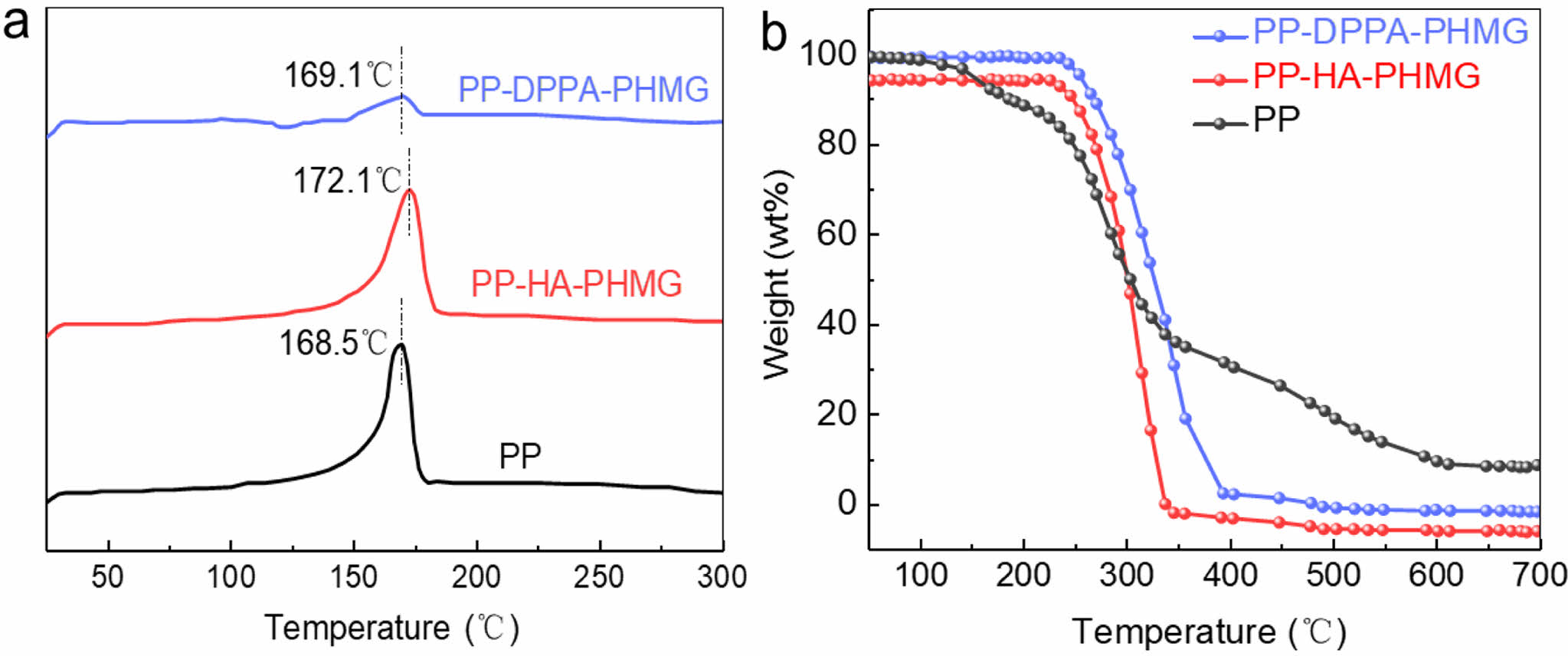
Figure 2 and Table 2 provided TG and DSC curves and data. The initial and maximum decomposition temperatures of PP-DPPA-PHMG and PP-HA-PHMG (289.8 ℃, 249.9 ℃ and 372.8 ℃, 369.6 ℃), were both higher than those of PP (223.7 and 359.9 ℃), indicating enhanced thermostability. The melting point of PP and PHMG are 168.5 and 156.00 ℃, respectively. After mixed with additives, the melting points of modified PP increased to 169.1 and 172.1 ℃ for DPPA-PHMG and HA-PHMG. The introduction of acid segments increased both molecular weight and combination strength of intermolecular interaction among polymer chains. Consequently, this structural modification endowed the molecular chains with greater combining strength during heating process, thereby enhancing thermo-stability.
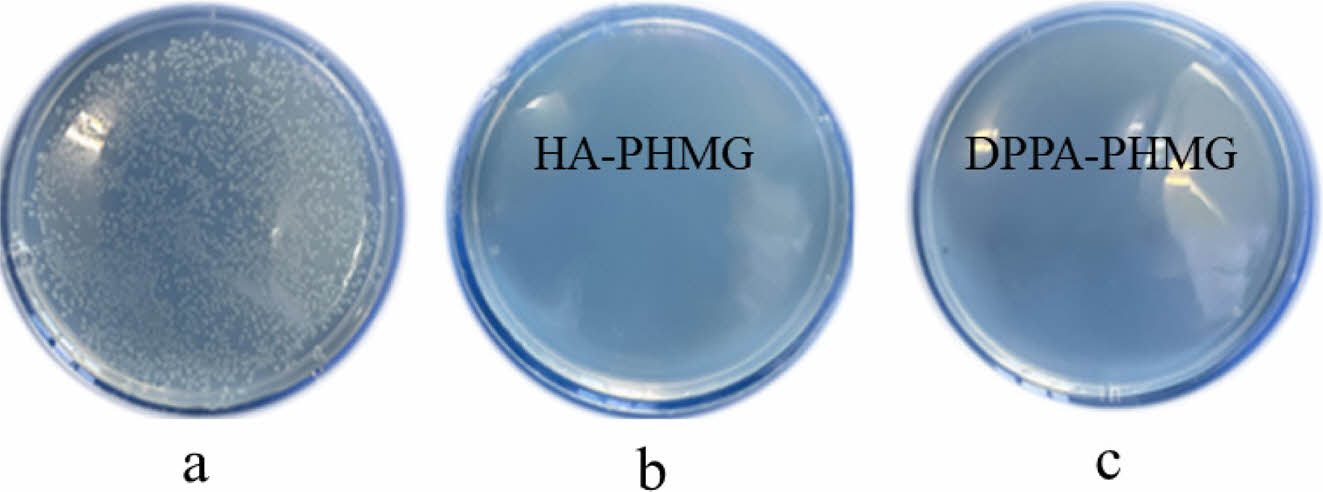
Antibacterial Performance of Membranes. Figure 3 was the digital photograph of petri dishes before and after 36h antimicrobial experiments. The pristine PP membrane did not exhibit any antimicrobial activity. Conversely, 0.02 g HA-PHMG or DPPA-PHMG provided 99.99% and 99.95% effective against E. coli. Both salts maintained antibacterial activity of PHMG, which confirmed that salinization reaction of -C=N-H in guanidine group with acids could not resist the dissociation of amine and imine groups into ammonium cations and did not affect antibacterial function of PHMG skeleton. Notably, HA-PHMG/PP membrane exhibits better bactericidal ability than DPPA-PHMG/PP, which was probably attributed to the multi-chlorine compound showing better antimicrobial activity.28
Flame Retardant Performance. Table 3provided combustion properties of samples. When the salt content was 1%, the splines ignited quickly accompanying with molten droplets. 3% HA-PHMG provided better flame retardant performance than 3% DPPA-PHMG. Molten droplets disappeared and smoke release and carbon residue increased at the same time. However, the deterioration of combustion performance of 5 wt% HA was attributed to the uneven surface under higher concentration, which in turn impacted its flame retardancy.29 The flame retardants would quickly form carbon layers in combustion process, which insulated heat transfer and O2 and hence slowed down burning speed. In conclusion, 3 wt% HA-PHMG showed superior flame retardancy, while DPPA-PHMG showed better performance when more than 5 wt%.
|
Figure 1 Characterization of smaples by: (a) FTIR; (b) 1 H NMR; (c) TG; (d) DSC spectra of PHMG, DPPA-PHMG, and HA-PHMG. |
|
Figure 2 (a) DSC; (b) TG curves of PP, PP-HA-PHMG, and PP-DPPA-PHMG masterbatches. |
|
Figure 3 Digital photography of petri dishes after antimicrobial experiments of E. coli by: (a) PP; (b) HA-PHMG-PP; (c) DPPA-PHMGPP membranes. |
Two composite functional additives, DPPA-PHMG and HA-PHMG were prepared and mixed with PP masterbatch, which were composed of both antibacterial and flame retardant parts. The antibacterial group, PHMG, endowed them excellent antimicrobial activity for solid polymers, such as PP, through the melting process. When combined with HA or DPPA, the salts obtained higher melting points and better thermal stability. They showed some flame retardant effect with 5% DPPA-PHMG and 3% HA-PHMG. Meanwhile, they decreased smoke emissions and increased carbon residue. It needs more work to improve flame retardant ability for the single additive, nevertheless, this work provided some ideas for developing multifunctional additives.
- 1. Liu, R.; Qing, X. C.; Feng, Z. B.; Zhang, X. M.; Zhang, Y.; Zhang, M. Y.; Zhu, B. Y.; Li, Y.; Xie, L. Research Status of Modification Methods for Antimicrobial Function of Grafting Polymer Materials. Polym. Bull. 2023, 36, 564-573.
- 2. Lv, T. J.; Zhang, X. D.; Gao, J. Q.; Liu, H. S.; Wang, J. Development of Plastics with Anti-bacterial Function. Liaoning Chemical Industry. 1999, 35-36.
- 3. Hou, X. J.; Xue, B. X.; Liu, S. H.; Zhu, Y. L.; Niu, M.; Yang, Y. Z.; Zhang, L. Antibacterial Properties and Biosafety of Silvered Silica Modified Silicone Rubber. Polym. Mater. Sci. Eng. 2023, 39, 111-116.
- 4. Tian, X.; Hua, T.; Poon, T.; Yang, Y. Y.; Hu, H.; Fu, J. M.; Li, J. H.; Niu, B. Study on Effects of Blending Fiber Type and Ratio on Antibacterial Properties of Chitosan Blended Yarns and Fabrics. Fibers Polym. 2022, 23, 2565-2576.
-

- 5. Zhang, Z.; Li, J.; Li, C. J.; Chu, L. Q.; Guo, M.; Wang, Y. Research Progress of Antibacterial Agents Used in Protective Fiber Materials and Their Antibacterial and Antiviral Mechanisms. Petrochemical Technology. 2023, 52, 1609-1618.
- 6. Liang, Y. Q.; Xu, J. X.; Ahmed, N.; Shi, J.; Wen, M. Y.; Yan, N. Construction of Biomass-based Flame-retardant, Antimicrobial and Hydrophobic Wood Coatings with POSS Organic-inorganic Nanoparticles. Eur. Polym. J. 2024, 219, 113370.
-

- 7. Fonseca, J.; Cano-Sarabia, M.; Cortés, P.; Saldo, J.; Montpeyó, D.; Lorenzo, J.; Llagostera, M.; Imaz, I.; Maspoch, D. Metal-Organic Framework-Based Antimicrobial Touch Surfaces to Prevent Cross-Contamination. Adv. Mater. 2024, e2403813.
-

- 8. Hemraz, U. D.; Lam, E.; Sunasee, R. Recent Advances in Cellulose Nanocrystals-based Antimicrobial Agents. Carbohydr. Polym. 2023, 315, 120987.
-

- 9. Gong, Y. T.; Xu, X.; Aquib, M.; Zhang, Y. H.; Yang, W. T.; Chang, Y. X.; Peng, H.; Boyer, C.; Whittaker, A. K.; Fu, C. K. Ammonium, Phosphonium, and Sulfonium Polymers for Antimicrobial Applications: A Comparative Study. ACS Appl. Polym. Mater. 2024, 6, 6966-6975.
-

- 10. Chen, Q.; Xu, Y. J.; Feng, J. Y.; Lv, X. S.; Fu, X. H.; Yuan, S. S. Hyperbranched Poly-L-Lysine Based Water-Insoluble Complexes as Antibacterial Agents with Efficient Antibacterial Activity And Cytocompatibility. Macromol. Biosci. 2023, 24, e2300388.
-

- 11. Qiu, W. Z.; Zhao, Z. S.; Du, Y.; Hu, M. X.; Xu, Z. K. Antimicrobial Membrane Surfaces via Efficient Polyethyleneimine Immobilization and Cationization. Appl. Surf. Sci. 2017, 426, 972-979.
-

- 12. Wang, Y. D.; Ma, L.; Wang, H.; Cheng, C. Z.; Yin, X. Z.; Zhu, Z. M. Fabrication of a Flame Retardant, Strong Mechanical Toughness and Antimicrobial Polylactic Acid by Chitosan Schiff Base/ammonium Polyphosphate. Polym. Degrad. Stab. 2023, 216, 110492.
-

- 13. Wang, Q. F.; Zhao, G. L. Investigation of Flame Retardant Modification and Thermal Degradation of Insulating Paper. Appl. Chem. Industry. 2006, 517-519.
- 14. Wan, C. Y.; Zhang, G. G.; Zhang, F. X. A Novel Guanidine Ammonium Phosphate for Preparation of a Reactive Durable Flame Retardant for Cotton Fabric. Cellulose. 2020, 27, 3469-3483.
-

- 15. Fang, Y. C; Chen, L. X; Liu, J. J; Wu, L. S. Multi-functionalization of Cotton Fabrics with Excellent Flame Retardant, Antibacterial and Superhydrophobic Properties. Int. J. Biol. Macromol. 2023, 254, 127889.
-

- 16. Seo, K. H; Lim, J. C. Phase Morphology and Foaming of Polypropylene/ethylene-octene Copolymer Blends. Polym. Korea. 2001, 25, 707-718.
- 17. Běhálek, L.; Dobránsky, J.; Pollák, M.; Borůvka, M.; Brdlík, P. Application of Physical Methods for the Detection of a Thermally Degraded Recycled Material in Plastic Parts Made of Polypropylene Copolymer. Materials. 2021, 14, 552.
-

- 18. Yang, X. L.; Liang, S. H.; Hou, Z. K.; Feng, D. L.; Xiao, Y.; Zhou, S. Z. Experimental Study on Strength of Polypropylene Fiber Reinforced Cemented Silt Soil. Appl. Sci.-Basel. 2022, 12, 8318.
-

- 19. Fu, Q. W. Characterization of the Crystal Structure of Polypropylene. Biochem. 2020, 3, 99-101.
- 20. Seifalian, A.; Basma, Z.; Digesu, A.; Khullar, V. Polypropylene Pelvic Mesh: What Went Wrong and What Will Be of the Future? Biomedicines. 2023, 11, 741.
-

- 21. Ömer Fırat TURŞUCULAR. A Review on Boron and Its Derivatives Used in Flame Retardant (FR) Applications for Cotton Fiber or Various Cotton Textile Fabric Forms. Textile Sci. Fashion Tech. 2024, 10, 000742.
-

- 22. Idumah, C. I. Emerging Advancements in Flame Retardancy of Polypropylene Nanocomposites. J. Thermoplast. Compos. Mater. 2022, 35, 2665-2704.
-

- 23. Lee, J. H.; Nam, J. H.; Lee, D. H.; Kim, M. D.; Kong, J. H.; Lee, Y. K.; Nam, J. D. Flame Retardancy of Polypropylene/montmorillonite Nanocomposites with Halogenated Flame Retardants. Polym. Korea. 2003, 27, 569-575.
- 24. Zheng, P. F.; Ping, G. S.; Li, F. T.; Zheng, H. G. Thermal Degradation and Flame Retardancy of Polypropylene/C60 Nanocomposites. Thermochim. Acta. 2008, 473, 106-108.
-

- 25. Choi, H.; Kim, K.-J.; Lee, D. G. Antifungal Activity of the Cationic Antimicrobial Polymer-polyhexamethylene Guanidine Hydrochloride and Its Mode of Action. Fungal Biol. 2017, 121, 53-60.
-

- 26. Sun, X. X.; Ji, J. H.; Zhang, W.; Wang, G. X.; Zhen, Z. C.; Wang, P. L. Guanidine-based Polymeric Microspheres with a Nonleaching, Antibacterial Performance. J. Appl. Polym. Sci. 2017, 134, 44821.
-

- 27. Yu, T. H.; Ji, F. F.; Zhou, Y. J.; Ding, R. Y. Standardized Assessment Methodologies for Evaluating Antimicrobial Efficacy in Textile Materials. (in Chinese). Text. Test. Stand. 2023, 9, 9-12.
- 28. Niro, A.; Pignatelli, F.; Fallico, M.; Sborgia, A.; Passidomo, F.; Gigliola, S.; Nacucchi, A.; Sborgia, G.; Boscia, G.; Alessio, G.; Boscia, F.; Addabbo, G.; Reibaldi, M.; Avitabile, T, Polyhexamethylene Biguanide Hydrochloride (PHMB)-properties and Application of an Antiseptic Agent. Eur. J. Ophthalmol. 2022, 33, 1724-6016.
-

- 29. Huang, Z. C.; Yang, Y. D.; Chen, L. J.; Tang, G. Preparation of BN@APP/Waterborne Polyurethane Composites and Their Flame Retardant Properties. China Plastics Industry. 2022, 1, 124-129.
- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(4): 411-416
Published online Jul 25, 2025
- 10.7317/pk.2025.49.4.411
- Received on Oct 31, 2024
- Revised on Jan 26, 2025
- Accepted on Feb 15, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Bowen Lv, Dongqing Liu
-
State Key Laboratory of Hollow Fiber Membrane Materials and Processes, Key Laboratory of Materials for Energy Storage, School of Material Science and Engineering, Tiangong University, Tianjin 300387, China
- E-mail: ldqnov@163.com
- ORCID:
0000-0002-3621-6319








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.