- Enhanced Enteric Soluble Drug Release Behavior and Stability by Shellac and Alginate Composite Films
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- 장내 용해성 약물 방출 거동 및 안정성 향상을 위한 셀락과 알지네이트 복합 필름
단국대학교 화학공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Shellac and alginate composites were prepared to enteric coating agent and obtained efficient drug release results. When shellac is used alone, low bending strength and an easily broken film are formed, but by adding alginate, the bending strength and hydrophilicity are improved. Also insoluble in acidic solution, and a composite that easily dissolves at a neutral pH can be obtained. The optimal shellac and alginate concentration was obtained through drug release, mechanical properties, and solubility tests under various concentrations. Drug release behavior and solubility were tested at various pH through absorbance of released dye and remaining weight, and the death of U-87MG cells was observed in a release test of disulfiram from composite. The composite prepared in this study will be able to be applied as a multi-purpose coating agent that can achieve the effect of surgical anti-adhesion barrier, tissue engineered skin, and releasing various drugs.
셀락과 알지네이트 복합재를 장용 코팅제로 제조하여 효율적인 약물 방출 결과를 얻었다. 셀락 단독으로 사용하면 낮은 굽힘 강도와 쉽게 깨지는 필름이 형성되지만 알지네이트를 첨가하면 굽힘 강도와 친수성이 향상되는 결과를 얻을 수 있다. 또한 산성 용액에 녹지 않으며 중성 pH에서 쉽게 용해되는 복합재를 얻을 수 있었다. 다양한 농도에서 약물 방출, 기계적 특성, 용해도 테스트를 통해 최적의 셀락과 알지네이트 농도를 얻을 수 있었다. 무게측정과 방출된 염료의 흡광도를 통해 다양한 pH에서 약물 방출 거동과 용해도를 테스트했으며, 복합재로부터 디설피람 방출 테스트에서 U-87MG 세포의 사멸이 관찰되었다. 본 연구에서 제조한 복합재는 수술 유착 방지 장벽, 조직공학적 피부 및 다양한 약물 방출 효과를 달성할 수 있는 다목적 코팅제로 적용할 수 있을 것이다.
Shellac and alginate composites were prepared to enteric coating agent and obtained efficiency drug release result and improved the bending strength and hydrophilicity.

Keywords: enteric coating, drug release, shellac, alginate, excipients.
The present research was conducted by the research fund of Dankook University in 2025
The authors declare that there is no conflict of interest.
Enteric coating is a polymer film that controls the location of oral medication in the digestive system where it is absorbed. Therefore, enteric coating is a common applied procedure in the development of orally administered dosage forms. The word “enteric” indicates small intestine; therefore enteric coatings prevent release of medication before it reaches the small intestine. For that reason, enteric coating is that it protects the drug from acidic pH and enzymatic degradation in the stomach while protecting it from the undesirable effects of some drugs.
The enteric coated polymers remain bound at low pH, and therefore remain insoluble. But as the pH increases in the small intestine, the acidic functional groups are capable of ionization, and the polymer swells or becomes soluble in the intestinal fluid.1-4
Various materials have been studied as Enteric coating materials.
Materials that are usually used in enteric coating include shellac, cellulose acetate phthalate (CAP), cellulose acetate butyrate, lipids (mixture of myristic acid, hydrogenated castor oil, castor oil, cholesterol, and sodium taurocholate), hydroxy propyl methyl cellulose succinate, and methacrylic acid co-polymers (Eudragit).5,6
Among these, shellac is a natural, nontoxic, biocompatible, and biodegradable polymer that is generally recognized as a safe substance by the FDA. The polymer is a resinous exudates from the Laccifer lacca insect inhabiting tropical forests. Shellac has been used for centuries in a variety of applications, including as a varnish, food glaze, and adhesive. Shellac is used extensively in the pharmaceutical industry as a coating material for tablets and capsules. The coating protects the drug from environmental factors such as moisture, light, and air, which can degrade the drug’s potency. Also, due to its stability in acidity is mostly used as enteric coating.7,8
However, shellac has a comparatively high dissolution pH of about 7.3 which is somewhat unsuitable for the application in conventional enteric coated dosage forms. Because of this high dissolution pH the addition of excipients is necessary to achieve a faster drug release in the small intestine.9,10
The excipients can include glidants (flow aids), diluents, binders or granulating agents and lubricants to ensure efficient tableting; disintegrants to promote tablet break-up in the digestive tract; sweeteners or flavours to enhance taste; and pigments to make the tablets visually attractive.11,12
As sodium alginate is extracted from the cell wall of brown seaweed it is a natural and plant based polymer. Moreover, sodium alginate is already established in the food industry as a thickener and coating agent.
The viscosity of alginate solutions increase as pH decreases, and reach a maximum pH around 3–3.5, as carboxylate groups in the alginate backbone become protonated and form hydrogen bonds.
Therefore, alginate has carboxyl groups which are charged at pH values higher than 3-4, making alginate soluble at neutral and alkaline conditions.13,14 Also, when both shellac and alginate are ionized, they are anionized, so it was expected that solubility and drug release effects would be increased by repulsive force.
In this study, to overcome the shortcomings of shellac, which exhibits drug release effects at a somewhat high pH, and to secure the physical stability of the film, a film-type enteric drug release matrix was prepared by using shellac as the main polymer and alginate as an excipient to increase drug release efficiency at neutrality, and We attempted to identify the drug release behavior and the response of brain tumor cells accordingly by applying disulfiram, which is known to pass through blood-brain barrier (BBB).15,16
Thus, the objectives of this study were i) to design enhanced enteric coating by the shellac and alginate composites; ii) to evaluate the enteric soluble drug release behavior and stability; and iii) to evaluate the glioblastoma (U-87MG) cytotoxicity by disulfiram Released.
Materials. In this study, shellac (ES Food Co, Gyeong-gi-do, Korea) was used as the base for the enteric coating resin. alginate (viscosity of 1% at 25 ℃ is 15-25 cps), zinc chloride, methanol, ammonium bicarbonate, the WST-8 assay kit, disulfiram, dimethylsulfoxide (DMSO), and cell culture reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals used were of analytical grade. Ultrapure water was obtained from a Milli-Q water system and used to prepare the aqueous solutions.
Fabrication of Shellac/alginate Composites and Measurement of Mechanical Properties. To prepare shellac solution, the polymer was dissolved during 3 h in 1.5% wt ammonium bicarbonate solution at 30℃ to a final concentration of 10% wt. And alginate solution of 3% wt was prepared by dispersion of the polymer powder in distilled water under stirring until complete dissolution. Each solution (shellac:alginate) was mixed at a volume ratio of 100:0, 80:20, 60:40, 50:50, and 30:70 at room temperature. 2000, 2480, 2780, 3080, and 3920 mL to match the total amount of polymer to 0.2 g were taken, applied on a glass slide and overhead projector (OHP) film having 75×26 mm, and then naturally dried for 3 weeks. Then, the excess of ammonium salt was then removed by heating the slides at 55℃ with 0.5N sulfuric acid, washed with excess water, and dried.
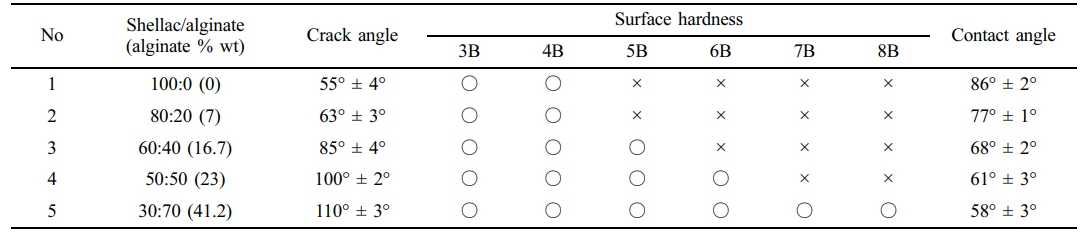
The purpose of this study is not to develop tough and strong materials, but to develop materials that can be implemented in various forms. Therefore, bending strength is an important property to implement various forms such as spherical, ribbon, sheet, and thread. For the crack occurrence test at various bending angles, shellac/alginate composite films coated on the overhead projector (OHP) films were prepared with composition shown in Table 1. A 5 N load cell with a crosshead speed of 1 mm/min (bending angle =180°) was used for this purpose. While bending the film for each angle, the film was visually checked for cracks, and the bending angle was measured when cracks occurred.
The surface hardness of the shellac/alginate composite films were determined by performing a hardness test using an Imoto IMC1552 pencil scratch hardness tester according to the JIS K5600-5-4 standard (equivalent to ISO/DIS 15184). In the pencil hardness test, pencils between 3B and 7B were used in consideration of the drug release film and the film strength stability in the digestive system.
The change in the water contact angle of the film surface according to the amount of alginate was measured. The contact angle was measured using a contact angle meter (Phoenix 150; Surface Electro Optics, Seoul, Korea).
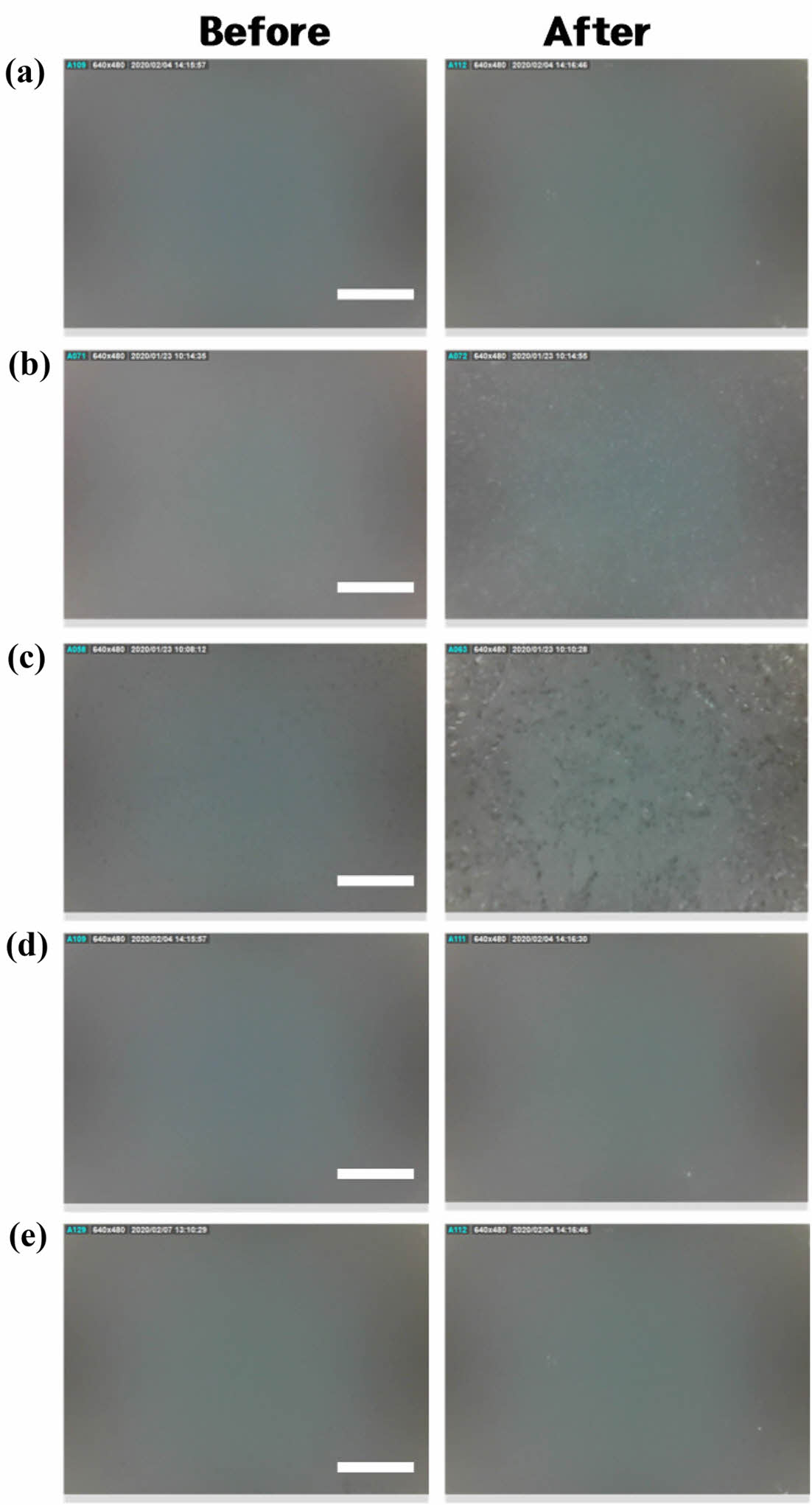
A digital microscope (A-2111; DinoLite, Taiwan) was used to measure the changes in the surface morphology of the film before and after dissolution for 90 minutes at pH 7.
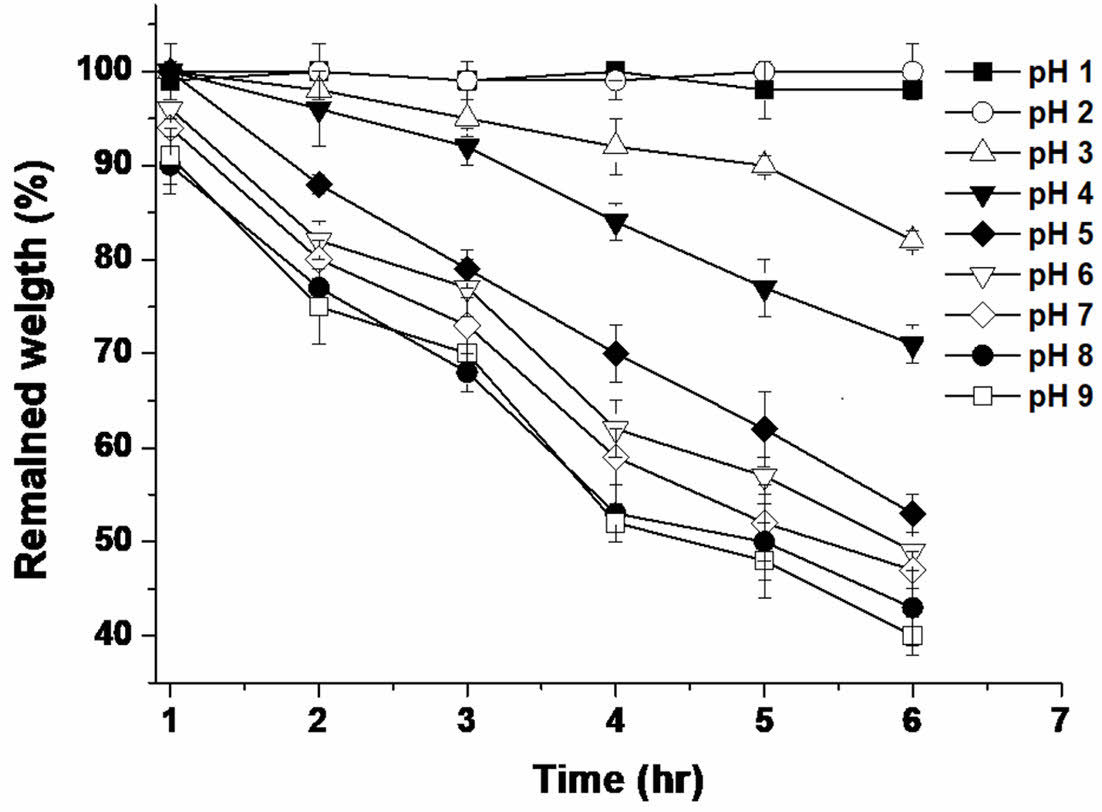
Determination of Solubility of Shellac/alginate Composites According to pH. Buffer solutions were used to prevent changes in the pH of the solution by eluted materials. 100 mL of pH 1, 2, 3, 4, 5, 6, 7, 8, and 9 solutions were prepared. Solutions of each pH were prepared in the following composition. pH 1, 2 (0.1 M, 0.01 M HCl), pH 3 (0.1 M sodium phosphate), pH 4, 5 (0.1 M sodium acetate), pH 6 (0.1 M sodium citrate), pH 7 (0.1 M sodium phosphate), pH 8, 9 (0.1 M Tris-HCl). The solubility of the 0.5 g of the pre-prepared dried shellac/alginate composite polymers (sample No. 4 in Table 1) in each pH under weak stress was studied by applying mechanical stress (10 rpm in solution; SH-802F incubator, Human Corp., Seoul, Korea) for 1, 2, 3, 4, 5, and 6 h at 37 ℃. Thereafter, the remaining weight of the dried films was measured using a precision electronic scale.
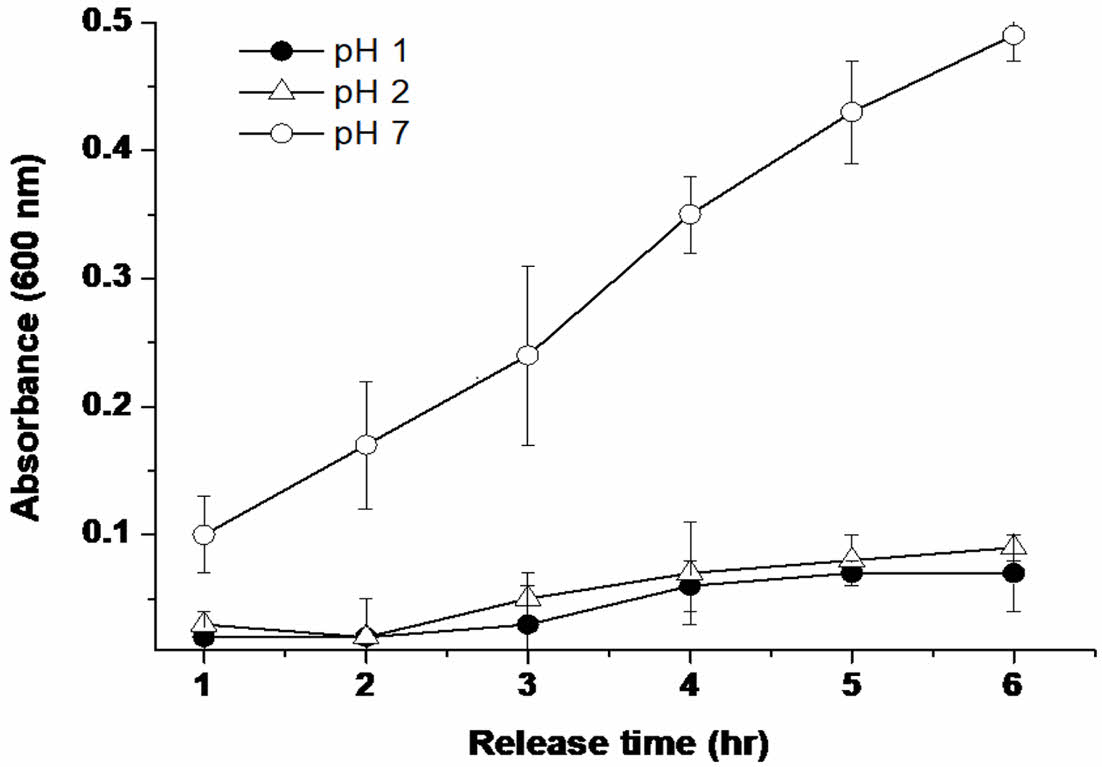
Dye Release Test From Shellac/alginate Composite Film. An important part of this test was the comparison of drug release ability in acid and neutral, such as pH 1, 2, and 7. Other data experimented with results at various pH, so the dye release experiment was conducted by choosing acid and neutral.
Drug release from the shellac/alginate composite film was simulated using the dye acid blue-25. In Table 1, acid blue-25 (0.5 mg/mL) was mixed with the composition of Sample No. 4 to prepare a film. The film was incubated and recovered at 37 ℃ at 50 mL of pH 7.0 PBS, pH 1 0.1 M HCl, and pH 2 0.01 M HCl solution for 1, 2, 3, 4, 5, and 6 h, concentrated, and put into a 96-well plate. The amount of the released dye was determined by measuring the absorbance at 600 nm using a microplate reader (Molecular Devices, Toronto, Canada).
Disulfiram Release Test and Cytotoxicity. Drug release from the shellac/alginate composite was also simulated using disulfiram. In Table 1, disulfiram (1 mg/mL) dissolved in DMSO was mixed with a composition of Sample 4 to prepare a film. The human U-87GM cells (30014, Korean Cell Line Bank, Seoul, Korea) were added directly to each plate in 200 µL of media (Dulbecco’s modified Eagle’s medium with 2 mM l-glutamine and 10% fetal bovine serum) suspension. The cell density was set at 2 × 104 cells per well. After 1 h, 1800 µL of the medium was added to each well and incubated at 37 ℃ and 5% CO2. Then, shellac/alginate/disulfiram composite film (20 × 20 mm) was incubated in the cell culture plate with U-87MG. The proliferation was quantified after 4, 24, 48, 72, and 96 h using a WST-8 assay.
Immunocytochemistry. The U-87MG cells in chamber slides were fixed with 4% formalin and incubated with a blocking solution containing 1% bovine serum albumin in PBS. The antibodies used in this study were as follows: anti-rabbit CD133 (Cat # PA5-38014, Thermo Fisher), Alexa 594 goat anti-mouse secondary antibody (Cat # A-11032, Invitrogen). The U-87MG cells were incubated with primary antibody for 2 h and subsequently with secondary antibody for 1 h. Further, the cells were stained with 4,6-diamidino-2-phenylindole (DAPI)-containing agent (Vector, Burlingame, Calif., USA), and were examined using a fluorescence microscope (BX-53, Olympus, Japan).
|
Table 1 Mechanical Properties According to Shellac/alginate Contents (n = 5) |
×: No scratch, ○: Scratch occurred. |
Mechanical Properties According to the Composition of the Composite. As shown in Table 1, As the amount of alginate increased in the shellac/alginate composites, the surface hardness and contact angle showed a tendency to decrease and increase the crack angle. In the case of a film with only shellac, the surface hardness was high, but the hydrophobic tendency tended to be strong, also it was easily broken even at low strength. On the other hand, as the amount of alginate increased, the surface hardness slightly decreased, while the angle at which crack occurred increased and the contact angle decreased by the ionized functional groups of alginate was observed. It is judged that the film strength lowered by alginate and the crack angle increased are the result of interfering with characteristics of the alginate itself with low molecular weight and the crystallinity of shellac when mixed with shellac. The purpose of this experiment is to study optimized drug release films rather than high physical strength. Therefore, as a potential optimum composition ratio, samples 3 and 4
were selected using the results of water contact angle and surface hardness.
Comparison of the Surface Condition Before and After Elution of Shellac/alginate Film in Neutral Solution. After immersing the films prepared by the composition of Table 1 in a neutral solution for 90 min, the elution state of the polymer was compared using a digital microscope. As shown in Figure 1, the film made only of 100% shellac did not observe any difference in the photos before and after elution, and when alginate was contained in 7% and 16.7% (Figures 1(b), (c)), it was observed that the surface was relatively unsmooth in the photos after elution. It is predicted that a small amount of alginate component is not well mixed with shellac at a neutral pH and the partially agglomerated part is eluted first. In addition, when 23% and 41.2% of alginate were contained (Figure 1(d), (e)), it was observed that the surface state was very evenly before and after elution. It is believed that when a certain amount of alginate or more was present, it was evenly mixed with the shellac and released with the shellac to the neutral solution. By combining this experiment and the physical characteristic test, it was determined that Sample 4 was the optimal composition, and an experiment was conducted on whether it led to actual weight reduction.
Solubility of Shellac/alginate Composite Film with Varying pH Solutions. The solubility and swelling properties of the shellac/alginate composite film are important physical properties in releasing drugs. Figure 2 shows the solubility of the shellac/alginate composite film in various pH solutions. No visible dissolution phenomenon was observed up to pH 3, whereas when the pH of the solution was higher than or equal to 4, it was observed that the solubility rapidly increased. Therefore, in this experiment, it was confirmed that the dissolution of the composition does not occur at pH 1 and pH 2, which are the same conditions as the acid in the stomach, but the dissolution phenomenon occurs visually at pH 4 or higher, and it can be used as a Enteric coating polymer film. According to previous research,14,17,18 the pKa of the shellac is about 6, and the pka of the alginate is about 3.7. Therefore, it is judged that the increase in the solubility of the composition was greatly influenced by the increase of the ionized alginate at pH 4 or higher, and it is judged that the alginate played a role in improving the solubility as an excipient even at a pH lower than the pka of the shellac.
Dye Release Behavior from Shellac Composite. The emission behavior of the dye was investigated under pH 1, 2, and 7. At pH 1 and 2, significant dye release from composite film was not observed, whereas drug release began to increase gradually by increased pH of the solution (pH 7) (Figure 3). According to a previous study,18 the pKa of shellac is approximately 6, and the pKa of the alginate is around 3.7. We hypothesized that dye release from shellac/alginate was due to ionized shellac and alginate at pH values greater than 7. In addition, even at pH 1 and 2, it was found that the drug was released somewhat over time, but this is judged to be the result of the swelling reaction due to the characteristics of the hydrogel. Therefore, it was confirmed that the drug release behavior can be controlled according to the pH, and a detailed cell investigation was conducted to confirm the effectiveness of the drug.
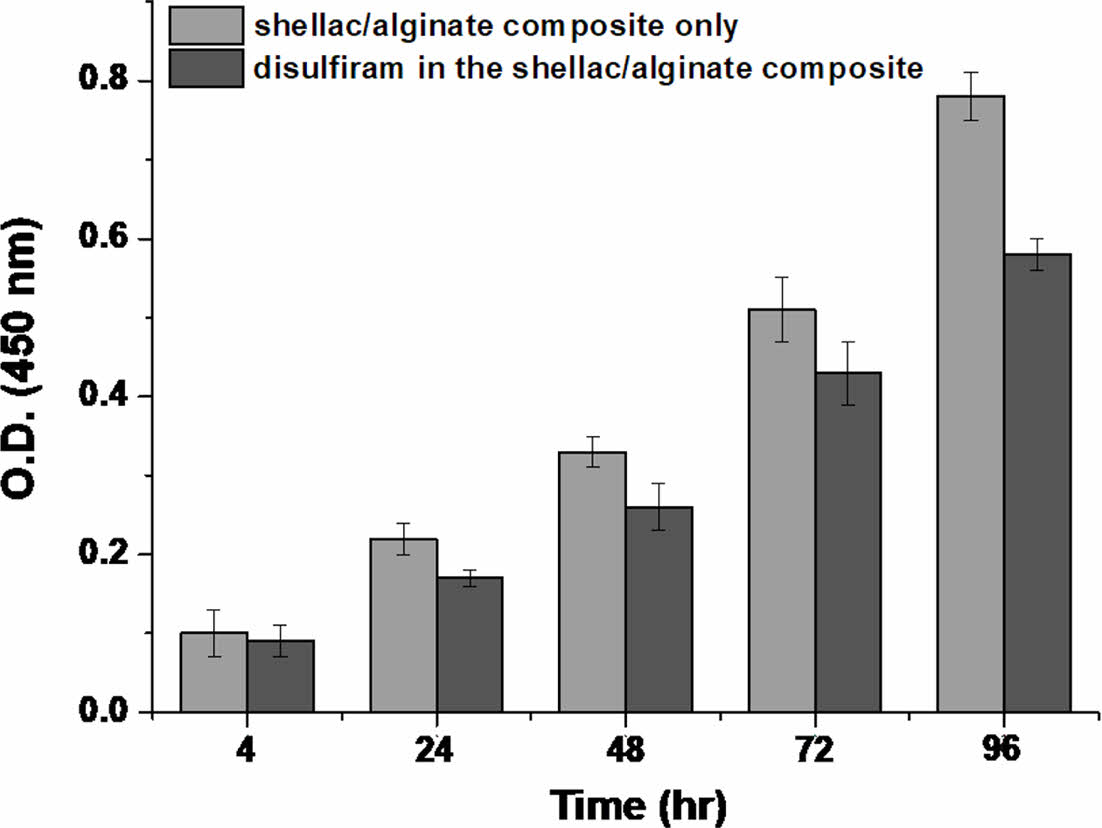
Cytotoxicity by Emission of Disulfiram from Shellac/alginate Composite Film. Cancer stem cells, the main cause of resistance and recurrence of cancer cells, express a lot of aldehyde dehydrogenase, and disulfiram is known to exert anticancer activity by activating the apoptosis mechanism by inhibiting aldehyde dehydrogenase. In addition, disulfiram has already been used as an alcohol poisoning treatment for decades, its stability has been proven, and it is known to pass through the blood brain barrier (BBB), recently, so research on its application as a treatment for brain tumors is also actively underway.19 While culturing U-87MG cells using a human glioblastoma cells (GBM), experiments were conducted on the release of drugs and cytotoxicity of the shellac/alginate composite film containing disulfiram. In addition, there is a result that disulfiram is activated in the presence of Zn2+ ions. However, in this study, we did not add it to prevent interaction with alginate. As shown in Figure 4, unlike the case where there is no drug, the overall cytotoxic effect was confirmed over time for the drug released in the neutral pH environment, after 96 h, the difference in cell viability was most clearly observed. Therefore, a more detailed study was conducted to see if it was the effect of the released drug.
Analysis of CD133 Positive Cells. Previous studies have reported that the proliferation of GBM cells was inhibited when aldehyde dehydrogenase was suppressed, and as cancer stem cells, there have been reports of reduced drug resistance by the suppression of CD133 positive cells.20,21 Therefore, immunochemical staining was performed to confirm the hypothesis of reduction of cell proliferation and cancer stem cells by synthesizing the previous results in this study. In Figure 5, immunochemical staining of CD133 of cells cultured in a drug-releasing state for 3 days was attempted. CD133 is the result of red fluorescent staining, and DAPI is the marker of blue staining of the nucleus of a typical cell. The degree of blue staining and red staining are reduced in the sample to which the drug is applied. Therefore, it was confirmed that the number of CD133 positive cells and surviving cells decreased. Through this experiment, it was confirmed that it was a result of the action of the released disulfiram.
|
Figure 1 Comparison of the surface condition before and after elution of shellac/alginate film in neutral solution using a digital microscope (×250, scale bar: 400 µm). |
|
Figure 2 Solubility of shellac/alginate composite film with varying pH (n = 5). |
|
Figure 3 Differences in the behavior of Acid blue-25 release from shellac/alginate composite film at 37 ℃ (n = 5). |
|
Figure 4 Differences in the cytotoxicity by disulfiram release from shellac/alginate composite film (n = 5). |

|
Figure 5 Results of immunostaining on CD133 positive cells. |
Enteric coating agent was prepared by a simple method using a shellac, alginate in aqueous solution. The bending strength, surface hardness, contact angle, drug release, and cytotoxicity of the composites were studied to determine the optimal composition for enteric coating agent applications. We found via mechanical property tests that 77% shellac, and 23% alginate was the most effective composition to increase the enhanced strength and drug release effect. In future research, it is required that more sophisticated drug release tests and mechanical tests, application of biological evaluation. Through this study, the shellac/alginate composite shows promise for use in enteric coating, and as membrane former in the medical field.
- 1. Singh, D. H.; Roychowdhury, S.; Verma, P.; Bhandari, V. A Review on Recent Advances of Enteric Coating. IOSR J. Pharm. 2012, 2, 5-11.
- 2. Mounica, P.; Pavani, S.; Mounica Rani, P. A Review on Recent Advances in Enteric Coating and Enteric Polymers. World J. Pharm. Res. 2018, 7, 475-495.
- 3. Chourasia, M. K.; Jain, S. K. Pharmaceutical Approaches to Colon Targeted Drug Delivery Systems. J. Pharm. Pharm. Sci. 2003, 6, 33-66.
- 4. Štefanič, M.; Locatelli, I.; Vrečer, F.; Sever, T.; Mrhar, A.; Bogataj, M. The Influence of Gastric Emptying Kinetics on the Drug Release from Enteric Coated Pellets in Fasted State: Anin vitro/in Vivocorrelation. Eur. J. Pharm. Biopharm. 2012, 82, 376-382.
-

- 5. Fang, Y.; Wang, G.; Zhang, R.; Liu, Z.; Liu, Z.; Wu, X.; Cao, D. Eudragit L/HPMCAS Blend Enteric-Coated Lansoprazole Pellets: Enhanced Drug Stability and Oral Bioavailability. AAPS Pharm. Sci. Tech. 2014, 15, 513-521.
-

- 6. Phothong, N.; Aht-Ong, D.; Napathorn, C. S. Fabrication, Characterization and Release Behavior of α-tocopherolacetate-loaded pH-responsive Polyhydroxybutyrate/cellulose Acetatephthalate Microbeads. Int. J. Biol. Macromol. 2024, 260, 129535-129550.
-

- 7. Maderuelo, C.; Lanao, J. M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability.Eur. J. Pharm. Sci. 2019, 138, 105019.
-

- 8. Lim, J. I. Drug-Releasing Type Teeth Anti-Aging Coating Agent Using Shellac and Polyvinyl Acetate Composites. Polym. Korea 2023, 47, 326-331.
-

- 9. Farag, Y.; Leopold, C. S. Development of Shellac-coated Sustained Release Pellet Formulations. Eur. J. Pharm. Sci. 2011, 42, 400-405.
-

- 10. Zhang, Y.; Man, J.; Li, J.; Xing, Z.; Zhao, B.; Ji, M.; Xia, H.; Li, J. Preparation of the Alginate/carrageenan/shellac Films Reinforced with Cellulose Nanocrystals Obtained from Enteromorpha for Food Packaging. Int. J. Biol. Macromol. 2022, 218, 519-532.
-

- 11. Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52-97.
-

- 12. Palou, A.; Cruz, J.; Blanco, M.; Tomàs, J.; de los Ríos, J.; Alcalà, M. Determination of Drug, Excipients and Coating Distribution in Pharmaceutical Tablets Using NIR-CI. J. Pharm. Anal. 2012, 2, 90-97.
-

- 13. Morales, E.; Rubilar, M.; Burgos-Díaz, C.; Acevedo, F.; Penning, M.; Shene, C. Alginate/Shellac Beads Developed by External Gelation as a Highly Efficient Model System for Oil Encapsulation with Intestinal Delivery. Food Hydrocoll. 2017, 70, 321-328.
-

- 14. Messaouda, G. B.; Sánchez-González, L.; Probst, L.; Jeandel, C.; Arab-Tehrany, E.; Desobry, S. Physico-chemical Properties of Alginate/shellac Aqueous-core Capsules: Influence of Membrane Architecture on Riboflavin Release. Carbohydr. Polym. 2016, 144, 428-437.
-

- 15. Zembko, I.; Ahmed, I.; Farooq, A.; Dail, J.; Tawari, P.; Wang, W.; Mcconville, C. Development of Disulfiram-Loaded Poly(Lactic-co-Glycolic Acid) Wafers for the Localised Treatment of Glioblastoma Multiforme: A Comparison of Manufacturing Techniques. J. Pharm. Sci. 2015, 104, 1076-1086.
-

- 16. Qu, Y.; Li, A.; Ma, L.; Iqbal, S.; Sun, X.; Ma, W.; Li, C.; Zheng, D.; Xu, Z.; Zhao, Z.; Ma, D. Nose-to-brain Delivery of Disulfiram Nanoemulsionin Situgel Formulation for Glioblastoma Targeting Therapy. Int. J. Pharm. 2021, 597, 120250.
-

- 17. Lim, J. I. Dual-functional Anti-adhesion Barrier Prepared Using Micro-hierarchical Structured and Neutralized Shellac Films for Drug Release. J. Biomater. Sci. Polym. Ed. 2020, 31, 2169-2181.
-

- 18. Yuan, Y.; He, N.; Xue, Q.; Guo, Q.; Dong, L.; Haruna, M. H.; Zhang, X.; Li, B.; Li, L. Shellac: A Promising Natural Polymer in the Food Industry. Trends Food Sci. Technol. 2021, 109, 139-153.
-

- 19. Veverka, K. A.; Johnson, K. L.; Mays, D. C.; Lipsky, J. J.; Naylor, S. Inhibition of Aldehyde Dehydrogenase by Disulfiram and Its Metabolite Methyl Diethylthiocarbamoyl-sulfoxide. Biochem. Pharmacol. 1997, 53, 511-518.
-

- 20. Silva, A. L.; Bai, S.; McLean, K.; Yang, K.; Griffith, K.; Thomas, D.; Ginestier, C.; Johnston, C.; Kueck, A.; Reynolds, R. K.; Wicha, M. S.; Buckanovich, R. J. Aldehyde Dehydrogenase in Combination with CD133 Defines Angiogenic Ovarian Cancer Stem Cells That Portend Poor Patient Survival. Cancer Res. 2011, 71, 3991-4001.
-

- 21. Huang, Y. K. Wang, T. M.; Chen, C. Y.; Li, C. Y.; Wang, S. C.; Irshad, K.; Pane, Y.; Chang, K. C. The role of ALDH1A1 in Glioblastoma Proliferation and Invasion. Chem.-Biol. Interact. 2024, 402, 111202.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(4): 458-463
Published online Jul 25, 2025
- 10.7317/pk.2025.49.4.458
- Received on Jan 6, 2025
- Revised on Feb 21, 2025
- Accepted on Feb 28, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusion
- References
Shared
 Correspondence to
Correspondence to
- Jin Ik Lim
-
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- E-mail: limjinik@dankook.ac.kr
- ORCID:
0000-0003-4803-0455









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.